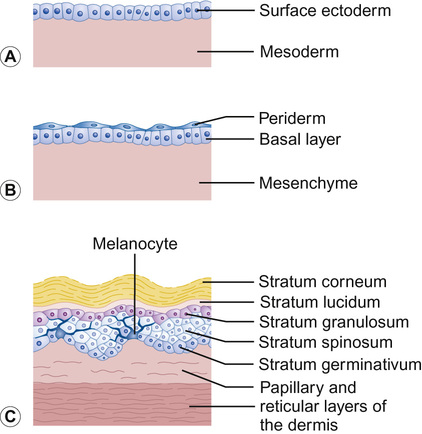
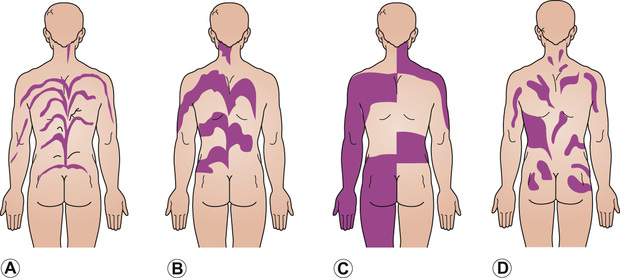
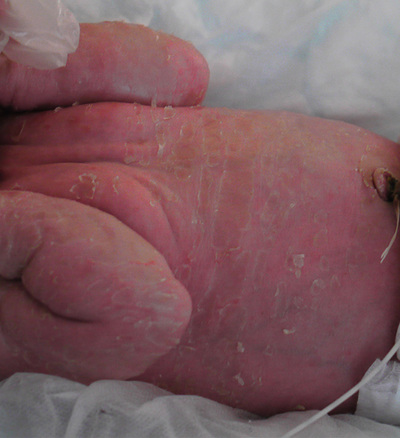
Nicole Y Z Chiang, Timothy H Clayton • Know the normal anatomy, physiology and function of skin • Know about dermatological history and examination • Know about the main dermatological investigations • Know about the pharmacology of agents used to treat common skin disorders • Know about associations between disorders of the skin and other systemic disorders Understanding the development of human skin offers an opportunity to study the structure and function of the skin in detail. It also helps to further the understanding of genetic skin diseases. The skin is divided into two distinct layers: the epidermis and dermis. Both layers are composed of various cell types that are derived from the ectoderm and mesoderm. The ectoderm that covers the developing embryo is initially a single-layered epithelium, the surface ectoderm (Fig. 25.1A). This layer proliferates and forms a layer of surface epithelium, the periderm (Fig. 25.1B), which covers the developing epidermis until the cornified cell layer is formed. The embryonic epidermis begins to stratify at approximately 8 weeks’ estimated gestational age (EGA), and is followed by cornification (composed of ‘dead’ keratinocytes held together by proteins and lipids). Until about 19 weeks’ gestation, the fetal skin is highly permeable and early in gestation, amniotic fluid volume is mostly determined by fetal surface area. At 19 weeks, keratinization occurs and the skin becomes impermeable. When differentiation is complete, the periderm detaches from the underlying epidermis, and its remnants form the vernix caseosa (greasy coat that protects the skin in utero from the amniotic fluid). The epidermis is morphologically similar to adult skin by mid third trimester (Fig. 25.1C). However, it does not acquire full barrier function until a few weeks after birth. Melanocytes are derived from the ectoderm. They migrate from the neural tube to the epidermis, and are not fully functional until the second trimester. Active melanocytes are present throughout the dermis during embryonic development, and most migrate to the epidermis or undergo apoptosis by the time of birth. When the melanocytes fail to reach their proper location in the epidermis and are entrapped in the dermis at the time of birth, this leads to the presentation of congenital dermal melanocytosis (Mongolian blue spot), the common blue-grey birthmark which slowly resolves spontaneously with time. Skin conditions that are a result of genetic abnormalities in the epidermis and/or its appendages often follow a distribution pattern (Fig. 25.2, Table 25.1) representing the migration pathways of epidermal cells during embryonic development. The pattern follows Blaschko’s lines (described by the German dermatologist, Alfred Blaschko) and represent a manifestation of cutaneous mosaicism. Cutaneous mosaicism occurs when two or more genetically different populations of cells exist side by side within the skin. They have typical patterns: V-shaped on the upper spine, S-shaped on the abdomen, linear on the arms and legs, spiral on the scalp and vertical in the mid-face. Many congenital and acquired skin conditions can follow Blaschko’s lines (see Table 25.1). An example of this is an epidermal naevus, which occurs as a result of a defect in ectoderm, leading to an overgrowth of the epidermal keratinocytes. It presents at birth or in early infancy, often with a localized, linear, warty, hyperpigmented plaque. Hypomelanosis of Ito (naevoid hypopigmentation) on the other hand, presents with hypopigmented streaks that follow Blaschko’s lines. Both conditions must be differentiated from incontinentia pigmenti, which is a neurocutaneous disorder syndrome due to a defect in the NEMO gene. This condition presents typically in the newborn with blisters that also follow Blaschko’s lines. The blisters then resolve and reveal hyperkeratotic, warty plaques, followed by increasing pigmentation at 2–6 months of age. The hyperpigmented brown streaks later fade into atrophic hypopigmented streaks in later childhood. These conditions may have associated extracutaneous manifestations, e.g. epidermal naevus, incontinentia pigmenti and hypomelanosis of Ito are associated with central nervous system, heart, eye, skeletal system and dentition defects, McCune–Albright syndrome is associated with bone and hormonal abnormalities, and focal dermal hypoplasia is associated with eye, musculoskeletal, renal, gastrointestinal, cardiac and neurological abnormalities. The mode of inheritance needs to be ascertained in order to provide appropriate genetic counselling for family members. Table 25.1 Patterns of cutaneous mosaicism Genetic abnormalities that result in a more global abnormal epidermal differentiation and barrier formation can give rise to the presentation of a ‘collodion baby’ (baby born encased in a taut, shiny, transparent membrane that is formed by aberrant stratum corneum) (Fig. 25.5). The membrane is shed over the first 2–3 weeks of life. In the majority of cases, the shedding will then reveal a phenotype of ichthyosis, a cornification disorder due to a genetic abnormality resulting in persistent dry and scaly skin. The origin of the dermis depends on the body site. The face and anterior scalp are derived from the neural crest ectoderm, whereas the extremities and trunk arise from the mesoderm. Dermal fibroblasts are developed under the epidermis by 6–8 weeks’ gestational age. These fibroblasts synthesize collagens and microfibrillar components. By 12–15 weeks, the distinction between papillary dermis and reticular dermis is formed. By the end of the second trimester, the dermis shifts from a non-scarring to a scarring form of wound repair. The dermal–epidermal junction (DEJ) provides adhesion between the basal keratinocytes and the dermis, as well as resistance against shearing forces on the skin. Mutations in genes that encode the components of the DEJ can result in skin fragility and blister formation. Strawberry birthmarks (infantile haemangioma) (Fig. 25.6) are thought to be a result of proliferation of endothelial cells and mutations in genes that encode for vascular endothelial growth factor (VEGF) and other pathways that affect the vascular development. They usually present shortly after birth (unlike vascular malformations – malformed dilated blood vessels in the skin, which are usually present at birth). Infantile haemangiomas are common in the head and neck area, and usually proliferate in the first 6–9 months. This is followed by spontaneous involution over several years and therefore active non-intervention is advised. However, treatment should be considered for lesions which are large with potential for disfigurement, ulcerating, threaten vital function, e.g. vision, hearing, breathing or feeding, or cause high-output cardiac failure. Until recently, steroids (topical, intralesional and oral) were the mainstay of treatment. However, the serendipitous discovery of a non-selective oral beta blocker as an effective treatment has revolutionized its management. Oral propranolol has become the treatment of choice for complicated lesions. The exact mechanism for its action is not completely understood, but is postulated to inhibit angiogenesis and recruitment of endothelial progenitor cells as well as inducing apoptosis. More recently, topical beta blockade in the form of eye drops (usually used for glaucoma) applied directly to the lesions has been shown to be effective. A number of genes are involved in the formation and migration of neural crest cells. Genetic mutations causing defects in the neural crest can lead to the development of neurocutaneous syndromes (i.e. disorders that affect the skin and are associated with CNS and other abnormalities). Examples of neurocutaneous syndromes include tuberous sclerosis, neurofibromatosis and Sturge–Weber syndrome (Table 25.2). Table 25.2 Neurocutaneous syndromes Autosomal dominant (50% have new mutations) (see Chapter 9, Genetics, for more details) Neurological abnormalities (e.g. seizures and cognitive impairment) associated with characteristic skin changes: Autosomal dominant (see Chapter 9, Genetics, for more details) Bony and/or neurological abnormalities, associated with characteristic skin changes: The development of the skin appendages (hair, nails, eccrine, apocrine and sebaceous glands) begins during the first trimester and matures in the second trimester. The epidermis is thought to convey signals to the dermis to initiate appendage formation. Abnormalities in epidermal development and the associated signalling pathways can result in skin appendage abnormalities. The epidermis is a stratified squamous epithelium. It has four or five layers: The skin epithelium changes and becomes super-specialized as the cells migrate to the skin surface, forming a squamous shape (elongated flattened cells). This is achieved by filling of the cytoplasm with proteins, especially keratin, and cross-linking these polymer fibres into strong stable networks. The squamous shape means the skin is resistant to mechanical trauma and allows shedding without disruption of the whole surface. The epidermal cells, known as keratinocytes, are held together by organelles called desmosomes. Desmosomes are cell adhesion structures that are especially prominent in the epidermis and mucous membranes. The basal cell layer is the source of new epidermal cells, and rete pegs ensure attachment of the dermis to the epidermis via the basement membrane (the multi-layered structure forming the DEJ). The epidermis consists of four major cell types: • Merkel cells – contain specialized nerve endings for cutaneous sensation • Langerhans cells – present antigens and active T-lymphocytes for immune protection The epidermal turnover time (migration of cells from stratum basale to stratum corneum) is approximately 30 days. The epidermis is supported and nourished by a thick layer of dense tissue underneath, the dermis. The dermis consists of a fibrous component (mainly collagen, and elastin) and ground substance (glycosaminoglycans), which are produced by fibroblasts. Collectively, they provide structural stability and elasticity to the skin. The dermis consists of two layers: papillary dermis and reticular dermis. It also contains vascular plexus supplied from vessels within the subcutaneous fat, and an extensive lymphatic and nerve system. The efferent system controls the cutaneous vasculature and its appendages, whereas the afferent system provides appreciation of cutaneous sensation. The dermis is attached to the underlying tissues by a layer of loose tissue called the hypodermis or subcutaneous layer, which contains variable amounts of adipose tissues. Sebaceous glands produce sebum via hair follicles (collectively called a pilosebaceous unit). They are most concentrated on the face and scalp. They are stimulated by androgens and become active at puberty. In general, sweat glands are divided into eccrine and apocrine glands. Eccrine glands are mostly concentrated on palms, soles, axillae and forehead. They regulate the body temperature. Apocrine glands are mainly found in axillae and perineal regions. They open into pilosebaceous follicles and produce body odour. The hair is made up of modified keratin. The hair cortex is produced from medulla within the hair bulb. The cortex contains densely packed keratin and is surrounded by a single layer of cells called cuticle. The hair colour depends on the amount of melanin in the cortex. There are three main types of hair: Most infants at term have a full scalp of terminal hair. However, shortly after birth, infants undergo a brisk period of shedding, where the pattern of synchronous hair growth shifts to the dyssynchronous hair growth of a normal adult. In an adult, approximately 85–90% of hairs are in the anagen phase, 10–15% in telogen and fewer than 1% in catagen. Congenital and hereditary hair disorders tend to present with abnormal pattern of hair growth or shaft morphology. Localized patches of hair loss (alopecia) may be a result of vascular compromise secondary to perinatal trauma, hamartomatous malformation, the development of naevi such as epidermal naevus and sebaceous naevus, or aplasia cutis. Generalized sparse hair may suggest an inherited structural hair defect or genodermatosis. In general, hair disorders can be broadly categorized into focal or diffuse, congenital or acquired and scarring or non-scarring (Table 25.3). The nail is made up of a nail plate (hard keratin) which arises from the matrix beneath the proximal nail fold. The nail plate rests on the nail bed, which contains blood capillaries that gives the pink colour of the nails. A white, crescent-shaped lunula extends from under the proximal nail fold. The lateral borders of the nail plate are enveloped by the lateral nail folds. The skin underlying the free end of the nail represents the hyponychium. Congenital and hereditary disorders often present with absence, hypoplasia (incomplete development), or dysplasia (abnormal development) of the nails. Congenital malalignment of the big toenail represents a lateral deviation of the nail plate from the longitudinal axis of the distal phalanx. As a result of repeated trauma, this congenital abnormality causes thickened nail plate, onycholysis (separation of the nail plate from the nail bed) and discoloration of the nail. With time, this can lead to an ingrowing painful toenail. Spontaneous improvement occurs in some patients. In ectodermal dysplasia, nails may be brittle, abnormally shaped, thickened, discoloured or absent. In pachyonychia congenita (an autosomal dominant disorder of keratinization), the nails usually appear packed and thickened (hyperkeratotic) and frequently discoloured.
Dermatology
Embryology
Embryogenesis of the skin
Epidermal development
Pattern of cutaneous mosaicism
Clinical example
Narrow bands of Blaschko
Incontinentia pigmenti, epithelial naevi, e.g. inflammatory linear verrucous epidermal naevus, hypomelanosis of Ito
Large bands of Blaschko
McCune–Albright syndrome
Chequerboard pattern
Becker naevus, vascular malformation (port wine stain)
Phylloid pattern (leaf-like)
Mosaic trisomy 13
Dermal development
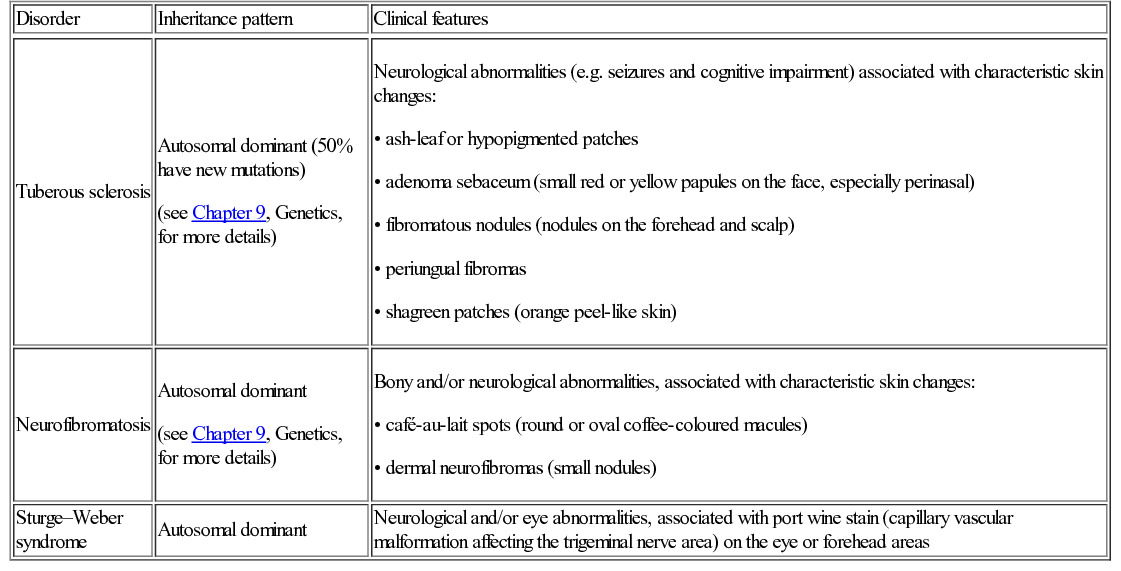
Disorder
Inheritance pattern
Clinical features
Tuberous sclerosis
Neurofibromatosis
Sturge–Weber syndrome
Autosomal dominant
Neurological and/or eye abnormalities, associated with port wine stain (capillary vascular malformation affecting the trigeminal nerve area) on the eye or forehead areas
Development of skin appendages
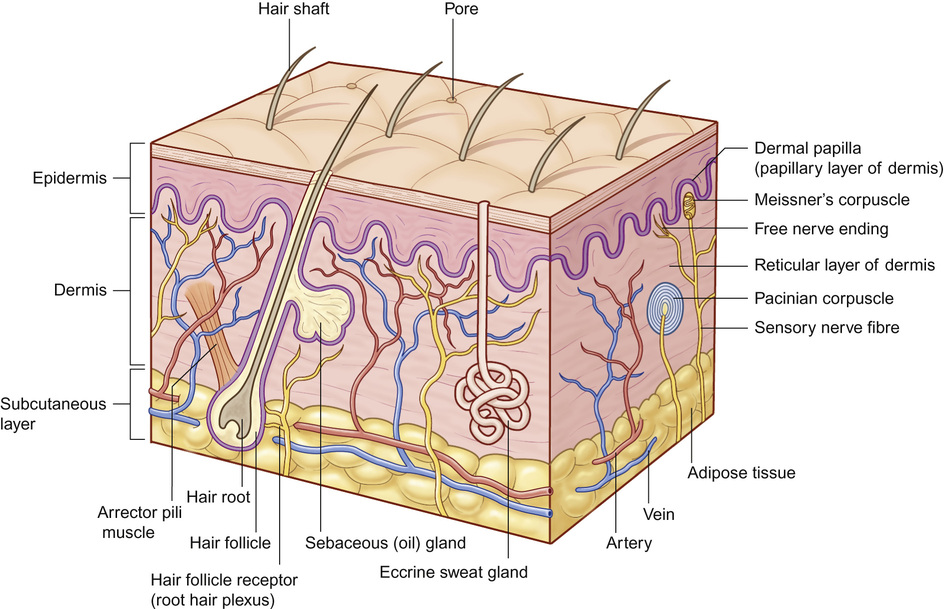
Anatomy of the skin (Fig. 25.7)
Epidermis
Dermis
Adnexal structures
Nail
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Dermatology
Chapter 25
Learning objectives
By the end of this chapter the reader should: