Background
Opioid use disorder among pregnant women is associated with adverse perinatal outcomes and is increasing in the United States. The standard of care for pregnant women with opioid use disorder is opioid maintenance therapy including either methadone or buprenorphine, which can be initiated at any time during pregnancy. These medications are known to cross the placenta but their placental and fetal effects have not been well characterized. Delayed villous maturation, a placental finding associated with stillbirth, was observed in placentas exposed to opioid maintenance therapy. Given the association of delayed villous maturation with stillbirth, and the possible relationship between opioid maintenance therapy and delayed villous maturation, this study was undertaken to explore the association between opioid maintenance therapy and this placental finding. Delayed villous maturation was not previously reported in placentas exposed to opioids or opioid maintenance therapy.
Objective
This study sought to compare risk of delayed villous maturation in term placentas exposed and unexposed to opioid maintenance therapy with buprenorphine or methadone.
Study Design
This was a retrospective cohort study conducted between 2010 through 2012 at Magee-Womens Hospital comparing delayed villous maturation in placentas of women with opioid use disorder exposed to either buprenorphine (n = 86) or methadone (n = 268) versus women without opioid use disorder (n = 978). Potential covariates were assessed in univariate analyses with none significantly associated with delayed villous maturation. The final model used conditional logistic regression adjusting for smoking status alone.
Results
Among women without opioid use disorder (and therefore not exposed to opioid maintenance therapy), delayed villous maturation was identified in 5.7% of placentas while the prevalence among women treated with buprenorphine or methadone was 8.1% and 10.8%. Overall, the crude odds of being diagnosed with delayed villous maturation were significantly greater in those exposed to opioid maintenance therapy compared to those not exposed (odds ratio, 1.86; 95% confidence interval, 1.20–2.89). When considered separately, women treated with methadone had significantly greater odds of having a placenta with delayed villous maturation than women without exposure to opioid maintenance therapy (odds ratio, 2.00; 95% confidence interval, 1.52–3.20). Women treated with buprenorphine did not have significantly greater odds of this placental diagnosis when compared to the women unexposed to opioid maintenance therapy (odds ratio, 1.46; 95% confidence interval, 0.64–3.31). Results were similar after accounting for smoking.
Conclusion
Delayed villous maturation was more common in the placentas of women exposed to opioid maintenance therapy. Further studies are required to characterize rates and extent of delayed villous maturation in the general population as well as to differentiate between possible effects of opioid exposure (eg, heroin, illicit use of prescription opioids) vs those of opioid maintenance therapy (buprenorphine and methadone).
Introduction
Opioid use disorder among pregnant women continues to rise in the United States. The recommended treatment in pregnancy is opioid maintenance therapy (OMT) with either methadone or buprenorphine. Pregnancies affected by opioid use disorder are known to have an increased risk of adverse perinatal outcomes including low birthweight, neonatal abstinence syndrome, neonatal mortality, postnatal growth deficiency, neurobehavioral problems, increased neonatal mortality, and sudden infant death syndrome. Treatment with OMT still carries a risk of neonatal abstinence syndrome but is preferred due to reduced rates of illicit drug use, improved birthweight, and increased likelihood of adherence to obstetric care. OMT is also thought to minimize adverse fetal effects by maintaining steady-state opiate concentrations in maternal and fetal blood thereby preventing repeated withdrawal episodes. These observations are based on limited data and further studies are needed to characterize the natural history of pregnancies affected by opioid use disorder and its treatment.
Methadone, an opioid agonist, and buprenorphine, a partial opioid agonist, are both known to cross the placenta and have been identified, along with their metabolites, in the placentas of exposed women. The effects of opioids on fetuses and placentas have not been well characterized, but have been associated with stillbirth. Anecdotal findings suggested an association between buprenorphine exposure in pregnancy and delayed villous maturation (DVM), a histologically diagnosed placental lesion associated with diabetes, aneuploidy, intrauterine growth restriction, and even perinatal death. A recent literature search for any associations between DVM and any maternal exposure, including medications, revealed no known relationship between DVM and any pharmacologic agent. DVM is a spectrum of placental disease that is characterized by decreased vascularization of the chorionic villi with a reduced number of syncitiocapillary membranes. Normal placental maturation involves transition of immature intermediate villi in the first and second trimester to mature intermediate and terminal villi in the third trimester. This transition results from an increasing number of peripherally placed, thin-walled vascular sinusoids and formation of vasculosyncytial membranes in villi, thereby shortening the distance between the maternal and fetal circulations and increasing the efficiency of gas exchange across the placenta. DVM, by contrast, involves a higher percentage of centrally placed villous vessels, a reduced number of vasculosyncytial membranes, and a longer travel distance between the maternal and fetal circulations. A reduced number of vasculosyncytial membranes, one of the principle histologic findings of DVM, theoretically prevents maternal supply from meeting fetal demand for oxygen and nutrients, and has been associated with stillbirth. This study was undertaken to explore the association between exposure to OMT in pregnancy and DVM.
We hypothesized that the prevalence of DVM would be more common in placentas exposed to either buprenorphine or methadone than in placentas unexposed to OMT. We hypothesized this association as both OMT and DVM are individually associated with stillbirth. We further hypothesized that placentas exposed to buprenorphine would have lower odds of DVM compared to those exposed to methadone given that previous studies associated buprenorphine with better neonatal outcomes.
Materials and Methods
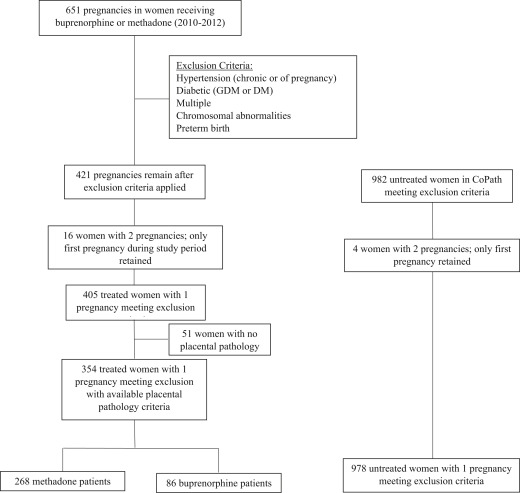
Magee-Womens Hospital, a component of the University of Pittsburgh Medical Center, is an urban tertiary care center with approximately 11,000 deliveries per year. After institutional review board approval was granted, claims data from our institution’s inpatient pharmacy were used to identify a cohort of women on OMT with either buprenorphine or methadone at the time of delivery from 2010 through 2012 ( Figure ). These dates were chosen based on availability of both maternal/perinatal data and pathology data within our perinatal registry. Pregnancies with claims recorded for buprenorphine or methadone at the time of admission for delivery were then identified within our Magee Obstetric Maternal and Infant Database to collect maternal demographics, comorbidities, and placental pathology records. Exposure was defined as any (yes/no) OMT at the time of delivery regardless of dose or length of treatment. We were unable to quantify length of treatment during pregnancy or severity of addiction. Only first pregnancies in the time period were retained. DVM was histologically diagnosed by placental pathologists (the majority by a single, specialized pathologist applying strict diagnostic criteria) based on the appearance of the villous structure (including amount of vasculosyncytial membranes, amount of stroma, and appearance of the trophoblast covering) and diagnoses recorded in the pathology report.
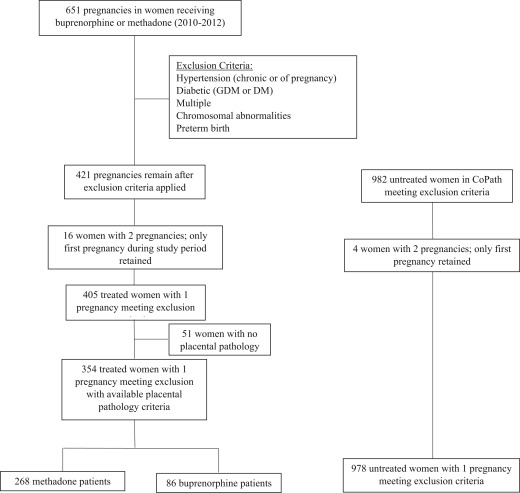
Women with diabetes (n = 32) and fetal aneuploidy (n = 3) were excluded as these diagnoses were associated with DVM. We also excluded women with hypertensive disorders (n = 60), multifetal gestations (n = 19), and preterm birth (n = 144) given that these diagnoses could be associated with abnormal vascular development in the placenta and could confound our results. Some women met >1 exclusion criterion. Our analysis was further limited to women with available placental pathology to capture a diagnosis of DVM. Overall, approximately 45% of placentas are sent for pathologic examination in our institution. Placentas sent for pathology evaluation are transported to pathology with a requisition identifying the indication for evaluation, and thus routinely reviewed in a nonblinded fashion. In our cohort of 405 women treated with OMT during pregnancy and meeting inclusion/exclusion criteria, 354 had available placental pathology (87.4%). For a control group we sampled 978 pregnancies not exposed to OMT that met our exclusion criteria and had placental pathologies available. Our final analytic sample consisted of 1332 women (methadone n = 268, buprenorphine n = 86, unexposed n = 978). Our primary outcome was the presence of DVM as documented in the placental pathology report. This approach to linking delivery data with pathology data was evaluated in our institution and found to be reliable, specifically in term deliveries with DVM found in the placenta. Data were compiled and analyzed in spring and summer 2016 using software (Stata, Version 13; StataCorp, College Station, TX).
Statistical analysis
Characteristics of women treated with buprenorphine or methadone and the control group were reported and compared. Univariate models were used to evaluate the effects of adjustment for maternal age, smoking status, and gestational age at delivery. Any covariate that was statistically significant at an alpha level of 0.05 remained in the final model. Maternal race was not included as a potential covariate as 96% of the treated women were Caucasian. Our analytic approach consisted of 2 steps. We compared the crude prevalence of DVM among placentas exposed to OMT to the rate in the unexposed control placentas with available pathology. Next, using logistic regression, we modeled the likelihood of DVM with the primary exposure as OMT. For each analysis, unexposed placentas were compared to those exposed to OMT in general (with either methadone or buprenorphine) as well as compared to placentas exposed to methadone and those exposed to buprenorphine (separated by specific treatment). No power calculation was performed as we analyzed a set sample inclusive of all women exposed in the available time period and the incidence of DVM in the general population has not been well characterized.
Materials and Methods
Magee-Womens Hospital, a component of the University of Pittsburgh Medical Center, is an urban tertiary care center with approximately 11,000 deliveries per year. After institutional review board approval was granted, claims data from our institution’s inpatient pharmacy were used to identify a cohort of women on OMT with either buprenorphine or methadone at the time of delivery from 2010 through 2012 ( Figure ). These dates were chosen based on availability of both maternal/perinatal data and pathology data within our perinatal registry. Pregnancies with claims recorded for buprenorphine or methadone at the time of admission for delivery were then identified within our Magee Obstetric Maternal and Infant Database to collect maternal demographics, comorbidities, and placental pathology records. Exposure was defined as any (yes/no) OMT at the time of delivery regardless of dose or length of treatment. We were unable to quantify length of treatment during pregnancy or severity of addiction. Only first pregnancies in the time period were retained. DVM was histologically diagnosed by placental pathologists (the majority by a single, specialized pathologist applying strict diagnostic criteria) based on the appearance of the villous structure (including amount of vasculosyncytial membranes, amount of stroma, and appearance of the trophoblast covering) and diagnoses recorded in the pathology report.