Cytomegalovirus
Mark R. Schleiss
Cytomegalovirus (CMV) is one of the family of eight human herpesviruses, designated as human herpesvirus type 5 (HHV-5) (Table 309-1). Taxonomically, it is referred to as a betaherpesvirus, based on its propensity to infect mononuclear cells and lymphocytes and on its molecular phylogenetic relationship to human herpesvirus type 6 (HHV-6) and human herpesvirus type 7 (HHV-7).  The proteinaceous layer between the envelope and the inner capsid, the viral tegument, contains proteins that are targets of host cell-mediated immune responses.
The proteinaceous layer between the envelope and the inner capsid, the viral tegument, contains proteins that are targets of host cell-mediated immune responses.
 EPIDEMIOLOGY
EPIDEMIOLOGY
Although most adults eventually become infected with cytomegalovirus (CMV), the epidemiology of this infection is complex, and the age at which an individual acquires CMV depends greatly on geographic location, socioeconomic status, cultural factors, and child-rearing practices.5-7 In developing countries, most children acquire CMV infection early in life, whereas in developed countries, the seroprevalence of CMV may be well below 50% in young adults of middle-upper socioeconomic status.
Transmission of CMV infection may occur throughout life, chiefly via contact with infected secretions. CMV infections in newborns are common, and most are subclinical. Approximately 1% (range 0.5–2.5%) of all newborns are congenitally infected with CMV. Most infections occur in infants born to mothers with preexisting immunity, and although clinically silent at birth, infection can lead to long-term sequelae, most notably, sensorineural hearing loss (SNHL).8 Additional information about congenital CMV infection is presented in Chapter 231. The route of acquisition of CMV infection acquired in utero is believed to be transplacental.9 CMV may also be transmitted perinatally, both by aspiration of cervicovaginal secretions in the birth canal and by breast-feeding.10 Toddlers are at high risk to acquire infection in daycare centers and may in turn transmit infection to their parents.11-13 In adults, sexual activity is a common mode of transmission.14 Although generally asymptomatic, heterophile-negative mononucleosis can be a presentation of primary infection in adulthood, as described later in this chapter.15 Blood transfusion-associated CMV was once an important cause of morbidity and mortality, notably in premature infants, but the routine use of leukofiltration has largely eliminated the problem of posttransfusion CMV.16
 PATHOPHYSIOLOGY
PATHOPHYSIOLOGY
In clinical specimens, one of the classic hallmarks of cytomegalovirus (CMV) infection is the cytomegalic inclusion cell. These massively enlarged cells (the property of “cytomegaly” from which CMV acquires its name) contain intranuclear inclusions that histopathologically have the appearance of “owl’s eyes” (Fig. 310-1). The presence of these cells indicates productive infection in vivo, chiefly in epithelial cells.
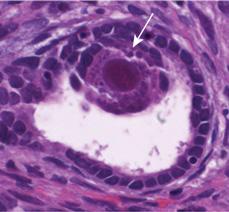
FIGURE 310-1. Characteristic cytomegalovirus (CMV) inclusion body in biopsy from congenitally infected infant. Classic “owl’s eyes” CMV-associated inclusion body noted in renal biopsy of infant with congenital CMV infection and (unrelated) renal disease. Viral inclusion found within renal tubule (arrowhead) in infant with high-grade DNAemia, viruria, and sensorineural hearing loss. Infant had no other stigmata of congenital CMV infection.
Little is known about the molecular mechanisms responsible for the pathogenesis of tissue damage caused by CMV, particularly for congenital CMV infection. Because CMV can infect endothelial cells, it has been postulated that a viral angiitis may be responsible for perfusion failure of developing brain with resultant maldevelopment. Others have postulated a direct teratogenic effect of CMV on the developing fetus. Observation of CMV-induced alternations in the cell cycle and damage to chromosomes support this speculation, although this hypothesis has been difficult to experimentally verify.17
Immunity to CMV is complex and involves both humoral and cell-mediated responses (reviewed in 18). As noted, envelope glycoproteins and tegument phosphoproteins are important in humoral and cellular immunity, respectively. More recent investigations into the molecular biology of CMV have revealed the presence of many genes that modulate the host immune responses (reviewed in 19). These include genes that inhibit MHC class I antigen presentation, homologs of cellular G-protein-coupled receptors, a homolog of the cellular major histocompatibility class I gene, homologs of chemokines, and a homolog of the tumor necrosis factor receptor super-family. These genes may contribute to the ability of CMV to escape immune clearance.
 CLINICAL MANIFESTATIONS
CLINICAL MANIFESTATIONS
Congenital CMV Infection
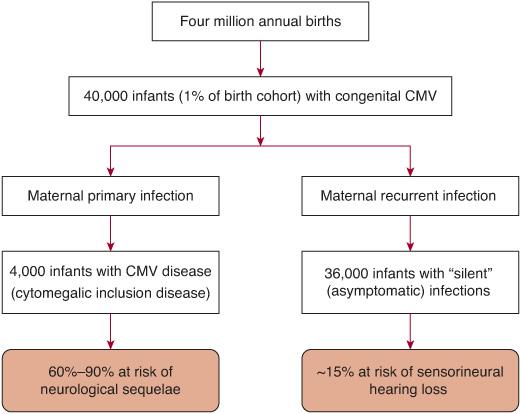
Current estimates suggest that 30,000 to 40,000 infants in the United States are born annually with congenital cytomegalovirus (CMV) infection. Approximately 10% of infants have clinical evidence of disease at birth (Fig. 310-2). The most severe form of congenital CMV infection is referred to as “cytomegalic inclusion disease” (CID), characterized by intrauterine growth retardation, hepatosplenomegaly, hematologic abnormalities (particularly thrombocytopenia), and cutaneous manifestations such as petechiae and purpura (so-called “blueberry muffin” baby).20,21 The most disabling manifestations of CID involve the central nervous system.22 Microcephaly, ventriculomegaly, periventricular calcifications, cerebral atrophy, polymicrogyria, cortical dysplasia, chorioretinitis, and sensorineural hearing loss are among the neurologic consequences (eFig. 310.1  ). Additional information on congenital CMV infection is presented in Chapter 231.
). Additional information on congenital CMV infection is presented in Chapter 231.
The majority of infants with congenital CMV infection are born to women who have preexisting immunity to CMV. These infants generally are clinically normal at birth but are at risk for neurodevelopmental sequelae, particularly sensorineural hearing loss. Routine newborn hearing screening may miss cases of CMV-associated hearing loss because hearing loss may not be evident until months or years after birth.23,24 Thus, a case can be made for universal newborn screening for congenital CMV infection.25-27
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


