Congenital Renal Masses
INTRODUCTION
Perinatally detected abdominal masses are a common clinical finding, and two-thirds originate from the kidney.1 With the increased use of prenatal ultrasound evaluation, approximately 15% of congenital renal masses are detected prenatally. Of those diagnosed postnatally, nearly half present with a palpable abdominal mass. Congenital renal tumors, however, are exceedingly rare, representing only 7% of all neonatal tumors.2 Abdominal ultrasound, whether done prenatally or postnatally, will often give an accurate description of these renal lesions. Congenital renal masses can be broadly divided into 2 main categories based on its sonographic appearance. Solid and cystic renal masses can be further subcategorized as either benign or malignant (Table 43-1).
Table 43-1 Differential Diagnosis of a Retroperitoneal Mass During the Neonatal Period
Congenital cystic renal masses are far more frequently encountered and more likely to be benign when compared to solid renal masses. The most common cystic lesions include hydronephrosis, multicystic dysplastic kidney, and hereditary polycystic kidney diseases (PKDs).3 The majority of congenital solid renal masses are also benign. Solid masses, however, can represent 1 of several rare neonatal neoplasms. During the first year of life, malignant Wilms tumor (WT) is the most common solid renal tumor, followed by congenital mesoblastic nephroma (CMN), rhabdoid tumor of the kidney (RTK), and clear cell sarcoma of the kidney (CCSK). However, during the neonatal period, two-thirds of all solid renal tumors are CMN, followed by WT, RTK, and CCSK4 (Table 43-1).
CYSTIC RENAL MASSES OF THE NEWBORN
Hydronephrosis
Fortunately, the great majority of congenital renal masses are benign. Forty percent of all neonatal abdominal masses are due to hydronephrosis and renal cystic disease.5 Depending on the onset and severity of hydronephrosis, a newborn child may warrant serial renal ultrasounds, voiding cystourethrograms (VCUGs), or renal scintigraphy in the early stages of life. The most common causes of antenatal hydronephrosis are ureteropelvic junction (UPJ) obstruction, ureterovesical junction obstruction, vesicoureteral reflux (VUR), primary megaureter, neurogenic bladder, posterior urethral valves, and prune-belly syndrome.3 These clinical entities are discussed in greater detail in the chapter 44 on obstructive uropathies.
Multicystic Dysplastic Kidney
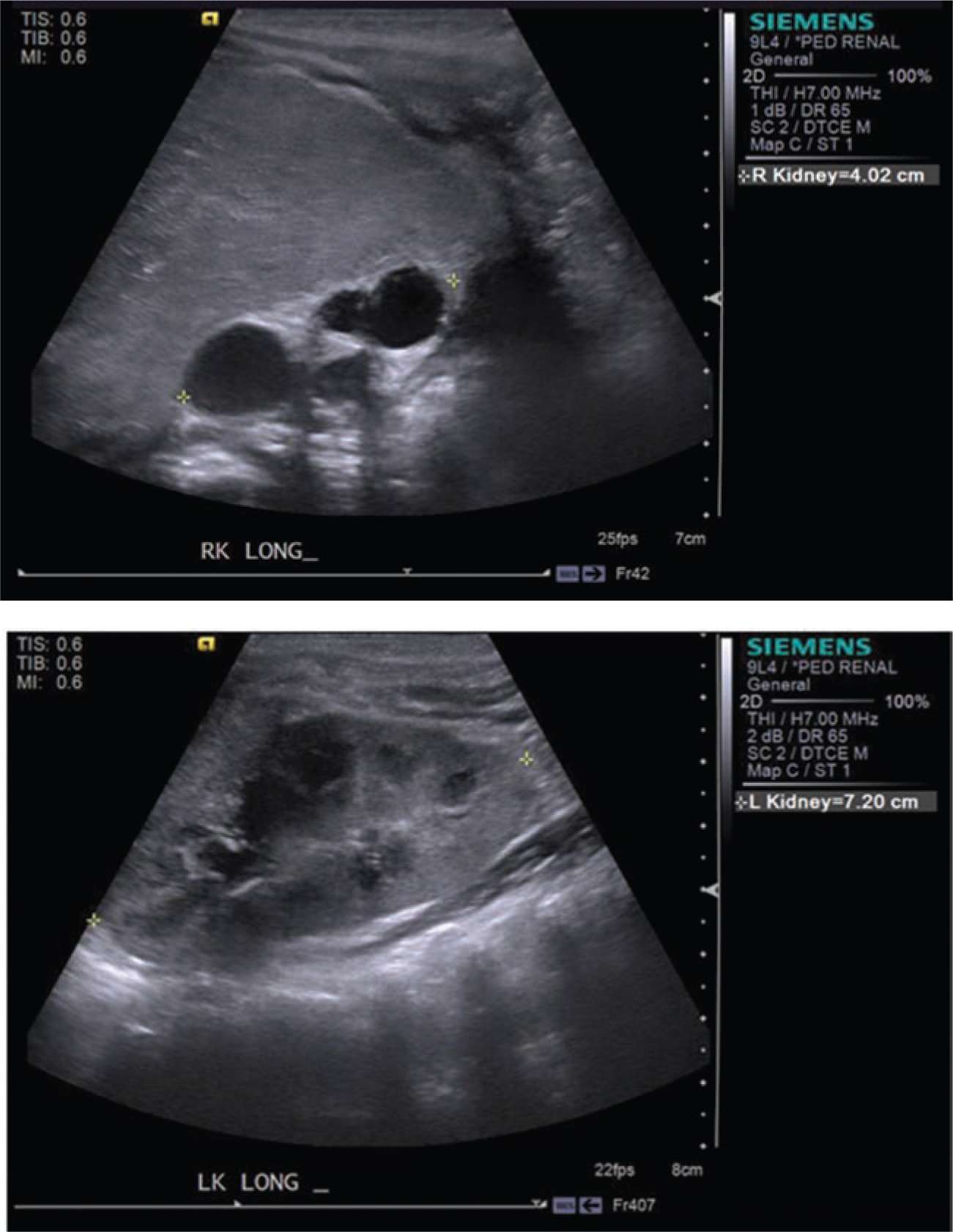
Unilateral multicystic dysplastic kidney (MCDK) is the most common cystic renal disease of the newborn, with an incidence of 1 in 4300 live births, occurring more frequently on the left-hand side.6 Some have postulated that MCDK is a result of ureteral obstruction or atresia,7 while others believe that aberrant or nonunion of the ureteric bud and metanephric blastema during the eighth week of gestation is the inciting event.8 This embryologic error results in a nonfunctioning cystic mass resembling a bag of grapes that is typically devoid of any nephrons and composed of primitive ducts and immature glomeruli.9 While a full description of MCDK can be found in chapter 44 on obstructive uropathies, Table 43-2 and Figure 43-1 outline the radiographic features that can help differentiate MCKD from hydronephrosis in the newborn.
Table 43-2 Radiographic Features Distinguishing Multicystic Dysplastic Kidney (MCDK) From Severe Hydronephrosis
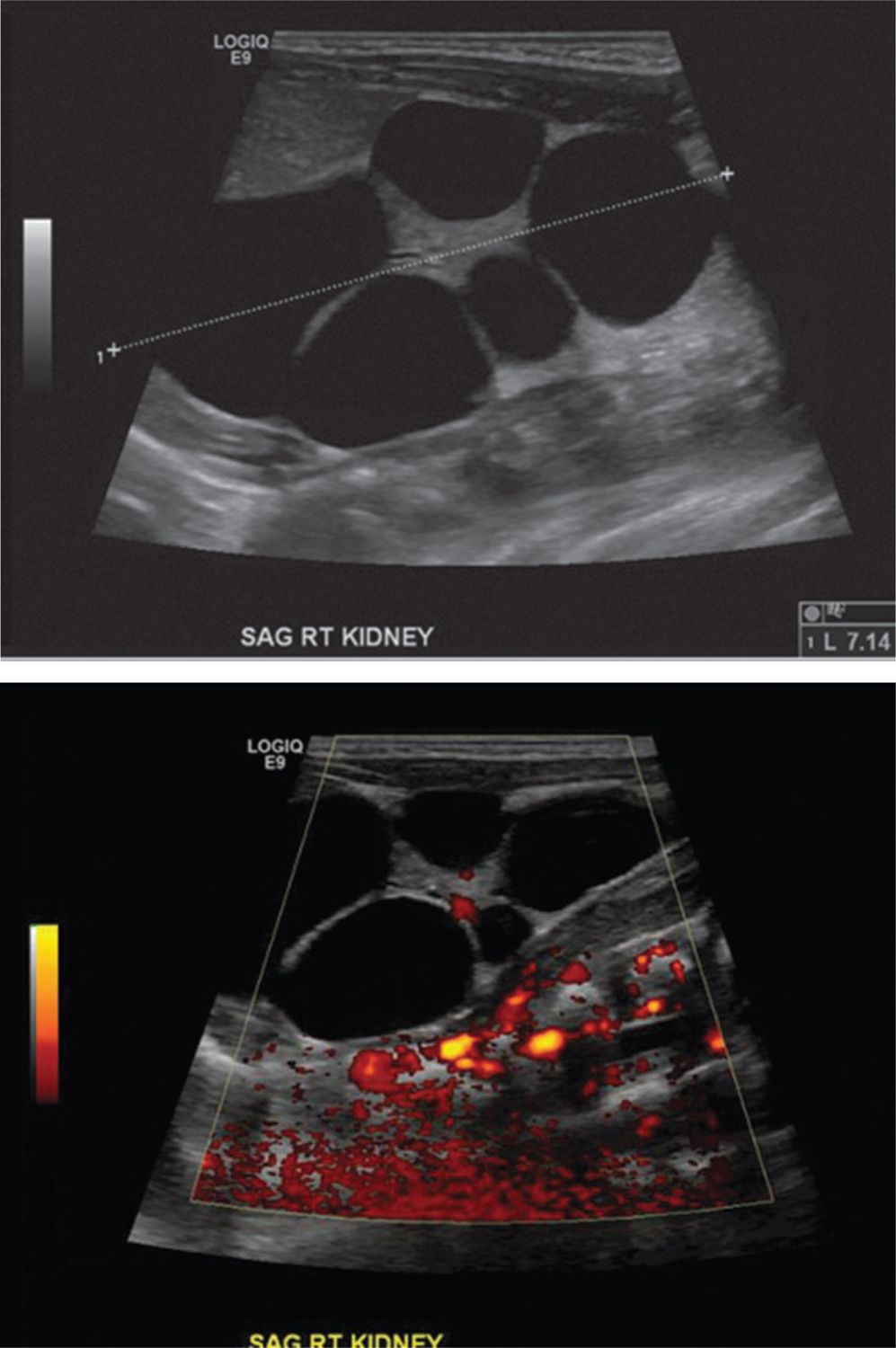
FIGURE 43-1
Polycystic Kidney Disease (Recessive Type and Dominant Type)
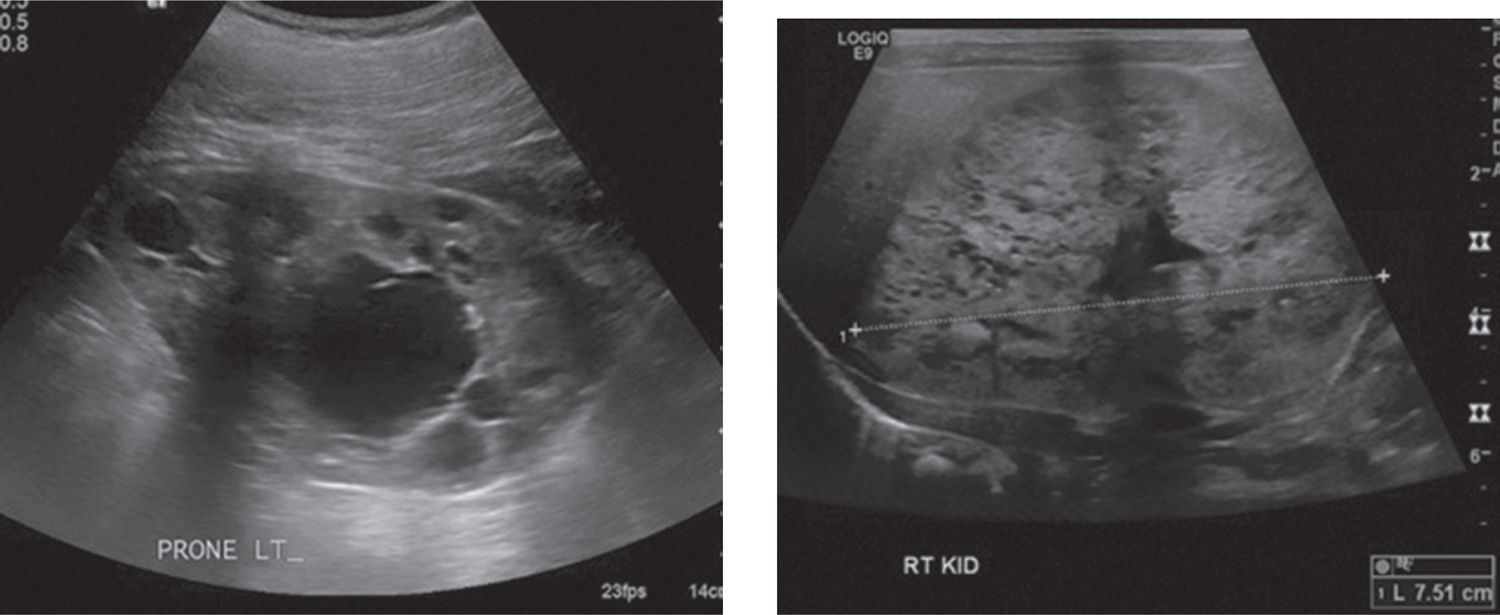
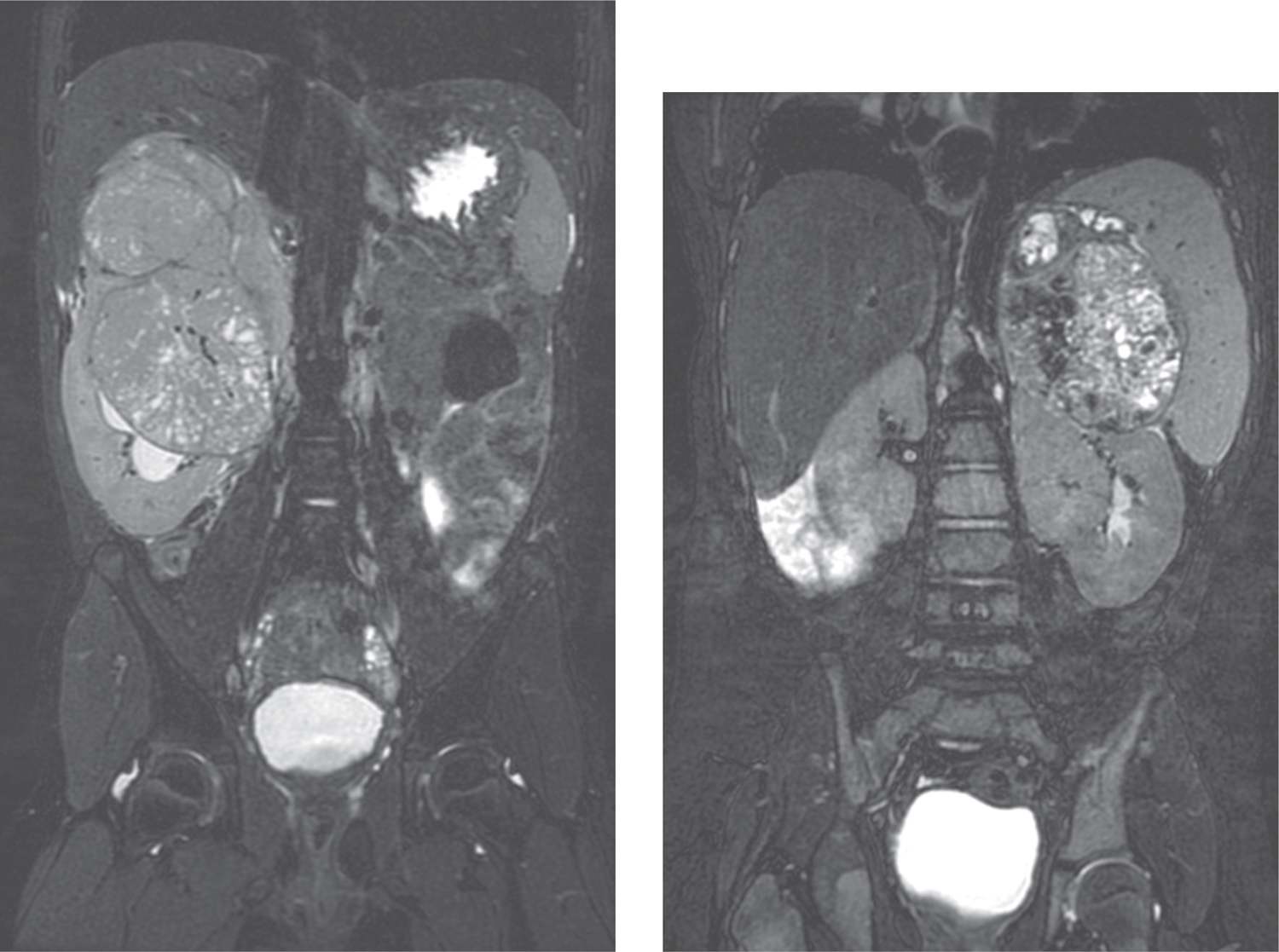
Polycystic kidney disease (PKD) can present at any stage of life, but when detected in the neonate, the recessive-type predominates.10 The severity of PKD varies with the age of disease onset and seems to appear in its most severe form earliest in life.3 It is important to note that the terms polycystic and multicystic differ despite implying the same thing. As explained previously, multicystic refers to a dysplastic kidney that underwent abnormal nephrogenesis and contains little-to-no nephrons, while polycystic refers to a nondysplastic kidney that developed normally and contains nephrons. Both autosomal recessive polycystic kidney disease (ARPKD) and autosomal dominant polycystic kidney disease (ADPKD) can present as a congenital renal mass. In both the fetus and the newborn, the diagnosis is considered when ultrasound examination reveals very large, homogeneously hyperechogenic kidneys bilaterally with loss of corticomedullary differentiation. Oligohydramnios is often present in utero. As seen in Figure 43-2, newborns with ADPKD demonstrate diffuse and large cysts, whereas ARPKD rarely contains visible cysts because the dilated collecting ducts are tightly packed together and too small to be resolved on ultrasound.3 Both ARPKD and ADPKD are discussed further in the chapter on acute renal failure in the newborn.
FIGURE 43-2
SOLID RENAL MASSES OF THE NEWBORN
Renal Vein Thrombosis
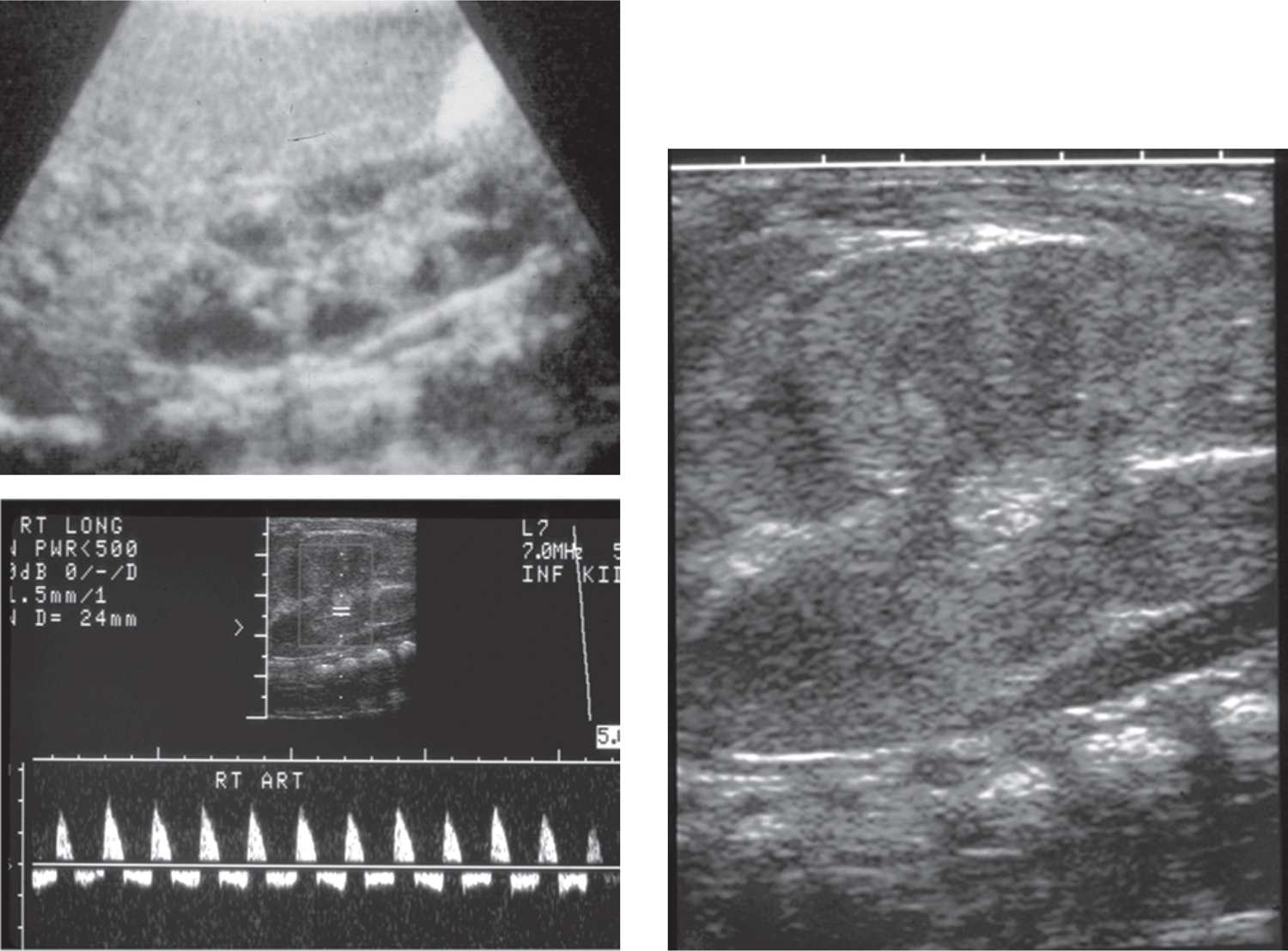
Renal vein thrombosis (RVT) is the most common non-catheter-related thrombotic event during the neonatal period. Two-thirds of all cases occur during the first 3 days of life, with only 7% detected in utero during routine ultrasound evaluation. There have been reports of in utero RVT being misdiagnosed as a congenital renal tumor and therefore must always be considered in a neonate with a renal mass.11 Renal sonography typically reveals an enlarged kidney that is focally or diffusely echogenic, with loss of the normal corticomedullary junction and occasional calcifications. The thrombus can be seen extending from the renal vein into the inferior vena cava in about half of cases. However, when only the intrarenal vessels are involved, a thrombus may not be visible.12 Doppler studies can be helpful in making an accurate diagnosis of RVT when the confirmatory thrombus is not visualized by demonstrating increased resistive indices and reversed diastolic flow through the renal vasculature.13 Approximately one-third of cases are bilateral, which often carries a grave prognosis for the child12 (Figure 43-3).
FIGURE 43-3
Horseshoe Kidney
Epidemiology
The horseshoe kidney (HSK) is the most common of all renal fusion anomalies, occurring between 1 in 400 and 1 in 666 people, or approximately 0.25% of the population, and has a 2-fold male predominance.14 A HSK consists of 2 distinct renal units fused at their inferior poles by a fibrous or parenchymal isthmus that crosses the midplane of the body and lies anterior to the vertebral column at the L3–L4 level (Figure 43-4). Rarely, in less than 5% of cases, the fusion occurs superiorly between the upper poles of the kidney.15
FIGURE 43-4
Pathophysiology
This anomaly takes place early on in fetal development, likely during the fourth and sixth weeks of gestation. Normally, after the ureteric bud and metanephric blastema fuse, they begin their rotational ascent to their typical locations high in the retroperitoneum. In the case of an HSK, a slight alteration in the orientation of the closely positioned fetal kidneys allows them to fuse. The fused metanephric mass then ascends en bloc until it is stopped in the midline by the inferior mesenteric artery as it exits the aorta, causing the horseshoe appearance. Due to the incomplete ascent, the HSK typically display aberrant vasculature with duplication or even triplication of the renal vessels, and the ureters often insert higher on the renal pelvis than in normal kidneys.16 Extra renal vessels and high-inserting ureters can cause partial obstruction at the UPJ, leading to dilation of the renal pelvis and obstruction of urinary flow. Distally, the ureters insert normally into the trigone of the bladder and are rarely ectopic.3
Diagnostic Tests
In most cases, the diagnosis of an HSK can be reliably made with ultrasound examination in both the antenatal and postnatal periods.17 The typical sonographic features of an HSK include low-lying kidneys bilaterally, malrotation with anterior-facing renal pelvises and posterior-pointing calyces, and ill-defined inferior poles. The most confirmatory sign is visualization of the isthmus, which can be seen sonographically in approximately 80% of cases.18 These features can be significantly altered due to various associated uropathies, such as hydronephrosis or multicystic dysplasia, making the diagnosis less clear. Historically, excretory urography was used to diagnose HSK, but this technology has been abandoned in lieu of newer and more reliable imaging modalities. When ultrasound evaluation is inconclusive, the diagnosis can be confirmed with computed tomography (CT), magnetic resonance imaging (MRI), or renal scintigraphy. Radionucleotide scans with technetium 99m dimercaptosuccinic acid (DMSA) have been reported to visualize the isthmus in 100% of children with HSK.19 Cross-sectional imaging studies are often reserved to accurately assess the renal anatomy, vasculature, and associated uropathies prior to surgical intervention.
Associated Anomalies
When diagnosed in the newborn, HSKs are frequently associated with additional congenital anomalies that tend to be less compatible with long-term survival. In an autopsy series that examined 99 cases of HSK, additional malformations were found in 79% of stillborns and infants who died during the first year of life. These fatal anomalies primarily involved the gastrointestinal, skeletal, cardiovascular, and central nervous systems.20 In another report, approximately one-third of all patients with HSK were found to have 1 or more additional congenital anomalies.21
Several chromosomal abnormalities have a predilection for HSK when compared to the general population. Trisomy 18 is associated with several renal anomalies, with HSK the most common and was seen in 21% of patients examined during an autopsy series.22 Turner syndrome is also commonly associated with renal anomalies, which primarily include HSK and ureteral duplication.23,24
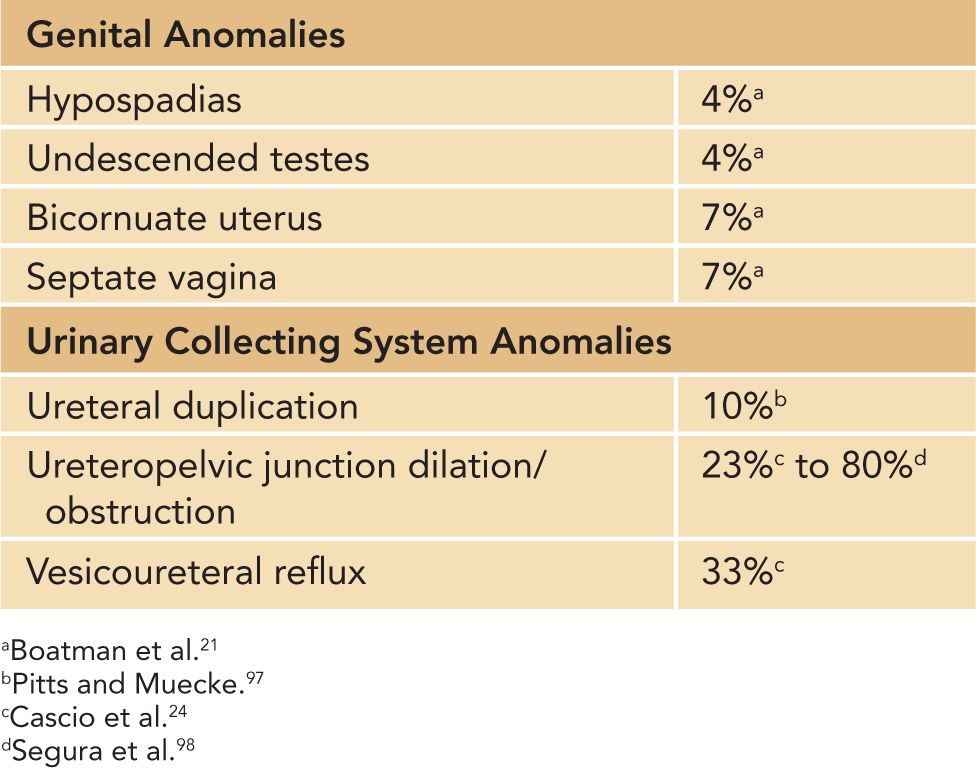
Genitourinary anomalies are frequently seen in patients with HSK (Table 43-3). VUR and UPJO are the most commonly encountered uropathies requiring surgical reconstruction.24
Table 43-3 Genitourinary Anomalies Associated With Horseshoe Kidneys
Management
In the newborn, there are no specific management recommendations for an HSK in an otherwise-healthy child. However, when associated congenital anomalies are present, supportive therapy may be required based on the degree and severity of their complications. Later in life, the most common indications for surgical management include UPJ obstruction, ZZVUR, kidney stone disease, and malignancy.17 Concomitant fetal uropathies, such as UPJ obstruction or VUR, should be investigated and managed accordingly, as outlined in chapter 44 on obstructive uropathies. They do not warrant any special treatment related to the presence of an HSK.
Outcome and Follow-up
It has been observed that over 60% of patients with an HSK remain asymptomatic over a 10-year period, and when symptoms occur, they are generally related to hydronephrosis, urinary tract infections, or nephrolithiasis.25
Congenital Mesoblastic Nephroma
Epidemiology
Congenital mesoblastic nephroma is the most common solid renal tumor of the newborn, accounting for 66% of tumors identified during the first 2 months of life. There is a slight predilection for males (male/female = 1.5:1).4 The proportion of CMN in relation to other congenital renal tumors is highest during the first month of life and quickly begins to decline as age increases, representing 54% of tumors diagnosed in the first month, 33% in the second month, 16% in the third, and less than 10% in the months thereafter.26 The true incidence of CMN is difficult to ascertain given its nonmalignant nature and lack of reporting to population-based cancer registries.27
Pathophysiology
Historically, CMN was classified as a congenital variant of WT but is now considered a distinct entity due to its almost-exclusive occurrence during the first year of life, nonaggressive behavior with low malignant potential, and mesenchymal origin.28 There are 3 histological variants: classic (49% of cases), cellular (30% of cases), and mixed (21% of cases). Classic histology tumors generally present earlier in life, with a median age at diagnosis of 17 days, compared to the mixed and cellular subtypes, which typically present after the neonatal period with a median age at diagnosis of 43 and 149 days, respectively.27 Classic histology CMN are nonmalignant and do not metastasize but may recur locally if incompletely resected. Despite the general benign nature of all CMN, the mixed and cellular variants have been reported to recur distantly after surgical excision29 and metastasize to the lung, liver, heart, bone, and brain.30
Recently, a chromosomal translocation at t(12;15)(p13;q25) has been identified in cellular CMN, which results in the ETV6-NTRK3 gene fusion (Rubin, Chen, et al, 1998). Infantile fibrosarcoma, another soft tissue tumor that generally behaves in a benign fashion despite aggressive histological features, shares this chromosomal translocation and establishes a genetic link between these neonatal neoplasms.31 Interestingly, the classic subtype does not express the ETV6-NTRK3 gene product, which has been used to subclassify these tumors based on molecular markers.32 However, the presence of this translocation does not clearly identify patients at risk of local or distant recurrence that may benefit from adjuvant chemotherapy.27
Clinical Presentation
Similar to all solid renal masses detected in the neonatal period, CMN most commonly presents with an abdominal mass detected by either physical examination or routine antenatal ultrasound. CMN are unilateral in nature and can be very large, affecting both left and right kidneys equally, and can result in hematuria (microscopic and macroscopic), nausea, and vomiting.4 When detected in utero, these masses are frequently associated with fetal and labor-related complications that increase perinatal morbidity and mortality. Polyhydramnios was detected in 39% of prenatally diagnosed cases of CMN and was associated with a significantly increased risk of preterm labor, resulting in a 50% rate of premature delivery. Nonimmune fetal hydrops, acute fetal distress, dystocia, anemia, and respiratory distress of the newborn requiring mechanical ventilation have all been described in these patients, which underscores the high-risk nature of these pregnancies.32 This strongly supports the recommendation for delivering children with antenatally detected renal tumors in a pediatric tertiary care center due to the high likelihood of perinatal complications that often require immediate surgical delivery and supportive therapy.
Congenital mesoblastic nephroma is also commonly associated with paraneoplastic syndromes. Hypercalcemia is secondary to a tumor-secreted parathyroid hormone-like peptide and occurs in less than 10% of cases. It can be severe enough to warrant supportive medical therapy prior to surgical resection and may require postoperative supplementation until the depleted bone reservoirs are replenished.33 Hypertension occurs in up to 22% of cases and is often related to elevated plasma renin activity and can thus be managed preoperatively with angiotensin-converting enzyme inhibitors.34 In all cases, elevated blood pressure and calcium levels normalized with complete excision of the mass.32
Differential Diagnosis
The differential diagnosis of a congenital solid renal mass is outlined in Table 43-1 and should include all benign and malignant intrarenal and extrarenal masses. During the neonatal period, the most likely diagnosis of a solid renal mass is CMN, followed by WT, RTK, and CCSK. There is no radiological finding or study that can reliably distinguish these lesions from each other. For this reason, all solid congenital renal masses are initially investigated and often treated in the same manner, with the final diagnosis based on pathological assessment from surgical specimens.
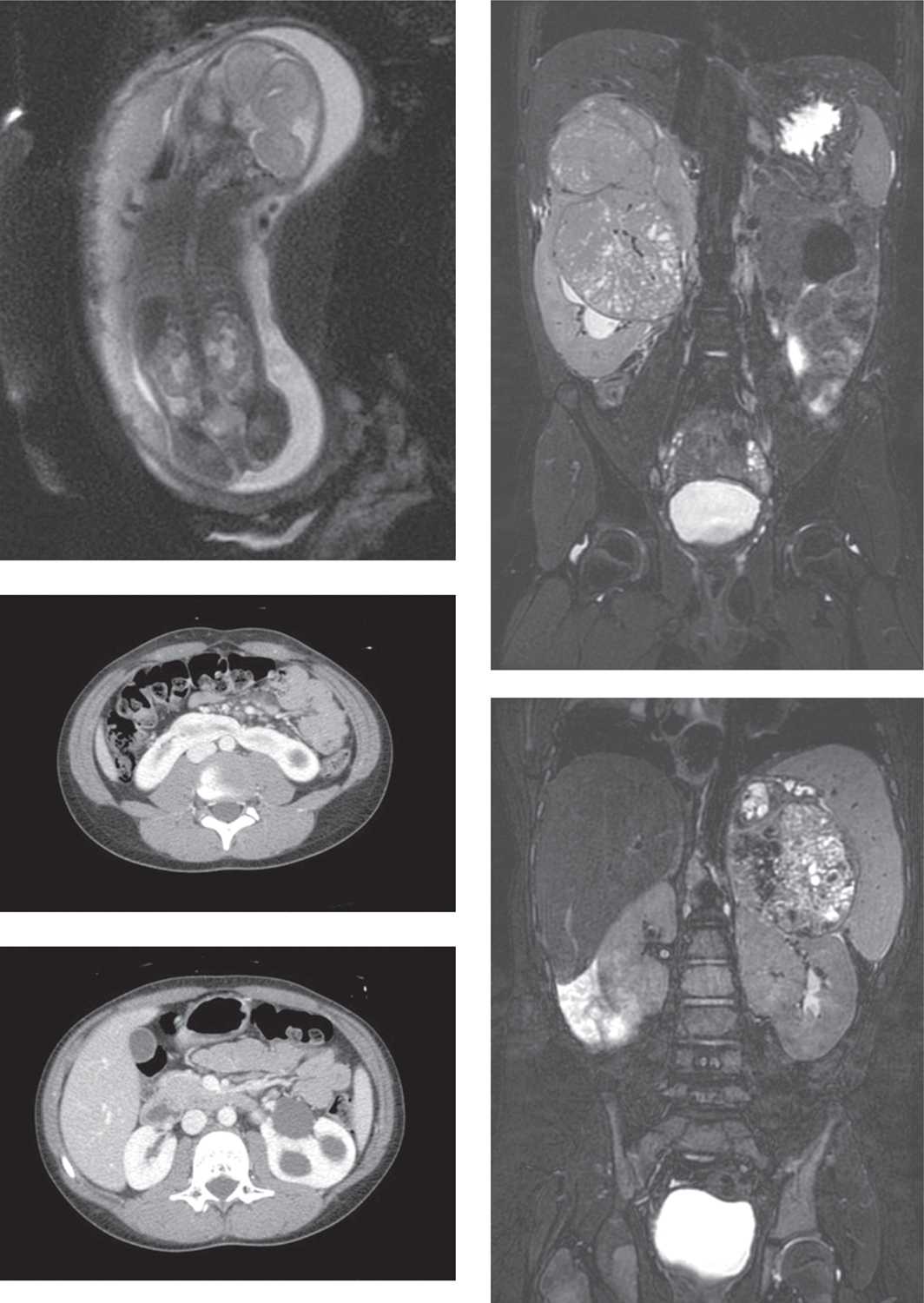
Extrarenal masses found in the retroperitoneum, including neuroblastoma and adrenal hemorrhagic cysts, can resemble an intrarenal mass arising from the upper pole and must also be considered in the differential diagnosis. During infancy, neuroblastomas are much more common than renal tumors and infiltrate the neighboring kidney through direct invasion in 20% of cases, mimicking a primary renal mass. However, cross-sectional radiologic studies can usually differentiate intrarenal tumors from neuroblastomas based on an adrenal or paravertebral sympathetic ganglion origin, the absence of the claw sign (renal parenchyma stretched around and cupping the tumor suggesting a renal origin), and a tendency for invasion rather than capsule formation35 (Figure 43-5).
FIGURE 43-5
Diagnostic Tests
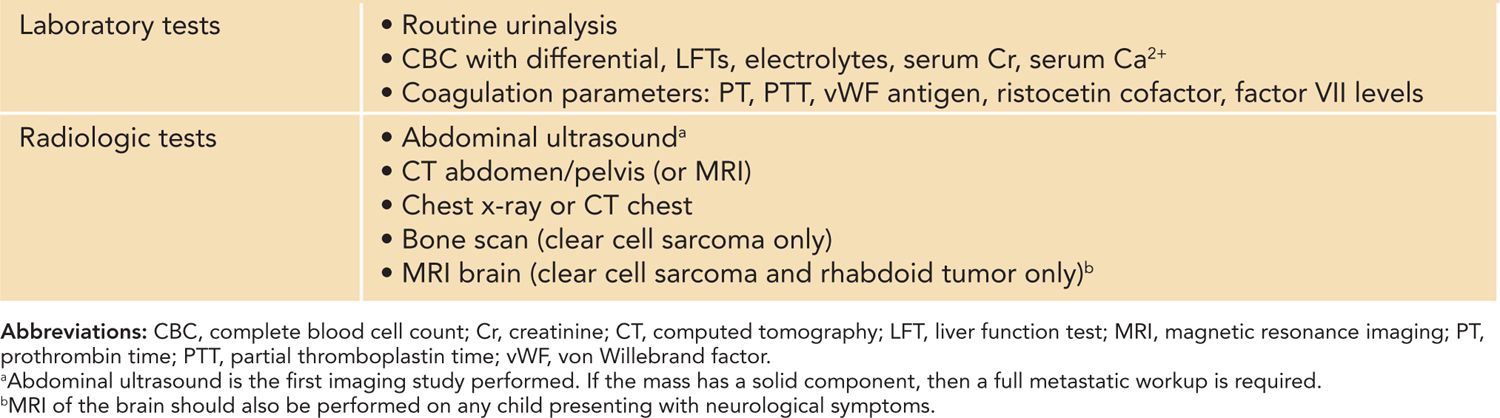
When investigating a newborn with a suspected renal mass, a thorough metastatic workup is required because of the potential for malignant pathology and includes both laboratory and radiological studies. Laboratory investigations are performed preoperatively and should include a complete blood count with differential, liver function tests, electrolytes, serum creatinine, serum calcium, and routine urinalysis. Acquired von Willebrand disease occurs in approximately 4%–8% of WT patients and has been reported in CMN,36 warranting the preoperative measurement of coagulation parameters (prothrombin time [PT], partial thromboplastin time [PTT], von Willebrand factor antigen, ristocetin cofactor, and factor VII levels) in most children prior to surgical resection37 (Table 43-4).
Table 43-4 Recommended Investigations for the Initial Workup of Neonatal Kidney Tumors
The radiologic studies performed should confirm the origin of the mass and establish whether there is any evidence of contiguous spread to the regional lymph nodes or inferior vena cava. In addition, the presence of a contralateral kidney should be confirmed and assessed for possible involvement, the urinary tract should be evaluated for congenital anomalies (ie, solitary, horseshoe, ectopic kidneys), and evidence of distant metastatic spread must be determined.
The initial imaging study of choice to evaluate a suspected neonatal renal mass is an abdominal ultrasound that focuses on both the kidneys and the bladder. On ultrasound, CMN appears as a large, solid, and heterogeneous renal mass that contains a cystic component in over 50% of patients. Cystic changes seen during sonographic evaluation correlated with cellular and mixed subtypes in over 80% of cases. CMN also often displays a hypoechoic ring that surrounds the periphery of the mass, which may help differentiate this mass from WT.38
Detection of a solid renal mass in a neonate mandates a full metastatic workup, which should include some type of contrast-enhanced cross-sectional imaging of the abdomen and, at a minimum, a chest radiograph. On CT, CMN appears as a large uniform mass with soft tissue attenuation that often replaces the majority of the kidney and occasionally extends beyond the renal capsule. Tumor extension into the renal vein has been reported but is exceedingly rare and should raise the suspicion of a WT.39 These masses generally show heterogeneous uptake of contrast material, with the tumor itself enhancing less than the neighboring renal parenchyma.40 Contrast-enhanced CT scanning of the chest provides the best means of detecting metastatic lung nodules as chest x-rays alone can miss a significant number of small lesions. These patients would therefore be understaged and receive suboptimal therapy, resulting in higher rates of recurrence.41 However, in this age category, pulmonary lymphadenopathy is often related to benign conditions, such as fibrosis or infection, and there is some degree of controversy regarding the clinical relevance of these lesions detected only by CT.
Magnetic resonance imaging is being used more commonly to evaluate congenital renal masses because it does not expose patients to ionizing radiation, and in some centers, it has replaced CT scanning as the baseline abdominal imaging study of choice.42 However, in this age group, MRI requires general anesthesia and is an inferior modality to assess the lungs for metastatic spread. MRI is the preferred imaging technique when assessing for intracranial metastases, typically seen with RTK and CCSK, and is recommended prior to initiation of treatment.26
Staging
There are 2 main staging systems used for pediatric renal tumors that were developed by the North American Children’s Oncology Group (COG) and the European Society of Pediatric Oncology (SIOP). The COG classification is based on pathological findings from up-front nephrectomy and lymph node specimens and preoperative imaging studies that assess for distant metastatic spread (Table 43-5). SIOP protocols generally treat with neoadjuvant chemotherapy followed by nephrectomy, and staging is therefore based on prechemotherapy imaging studies and postchemotherapy surgical findings.35
Table 43-5 Children’s Oncology Group Staging System for Pediatric Renal Tumorsa
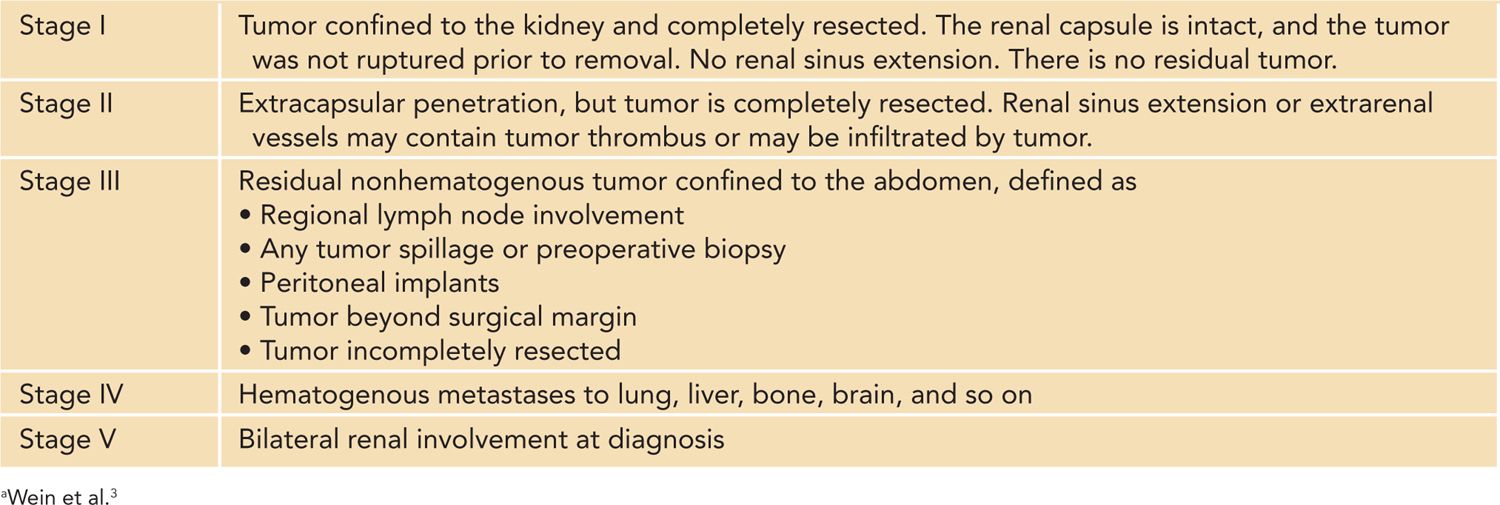
In the largest North American series examining fetal and neonatal renal tumors, CMN typically presented with low-stage disease, and 88% of all cases were classified as stage I/II. Stage III and IV disease was found in 9% and 3% of cases, respectively, with no reports of bilateral or stage V tumors during the neonatal period.4
Management
The initial management of a neonatal renal tumor requires a multidisciplinary approach in which complete excision of the mass is paramount. During the neonatal period, standard therapy for a unilateral solid renal mass includes a radical nephrectomy with mandatory regional lymph node sampling via a transabdominal approach.3 It is critical that the surgeon adhere to basic oncological principles due to the potential for malignant pathology and perform a complete en bloc excision of the mass and involved structures with careful handling of the mass to avoid tumor rupture and spillage. A formal retroperitoneal lymph node dissection is not recommended due to the morbidity associated with the procedure in the absence of any prognostic benefit.43 Partial nephrectomy is not recommended because CMNs have a tendency to grow into the renal sinus and perirenal fat, increasing the risk of local recurrence.44
Postoperative complications are common and include hemorrhage, small-bowel obstruction, chylous ascites, and intussusception. Significant cardiovascular complications have also been reported, including intraoperative hypertensive crisis and cardiac arrest.27
In the setting of classic CMN, surgery alone has been associated with excellent outcomes, and there is no current role for adjuvant therapy with complete excision of the mass.39
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree