Congenital and Perinatal Human Cytomegalovirus Infections
EPIDEMIOLOGY OF CONGENITAL HUMAN CYTOMEGALOVIRUS INFECTIONS
Introduction
The natural history of congenital human cytomegalovirus (HCMV) infection has been studied exhaustively for nearly 4 decades, yet critical features of this intrauterine infection continue to be redefined as contemporary methodologies are employed to further investigate the biology of the virus and the disease. Perhaps this is most strikingly illustrated by the surprising findings that have been obtained from the application of next-generation nucleic acid sequence technologies to studies of the genetic diversity of viruses isolated from infants with congenital infections.1 These studies have confirmed earlier studies that utilized comparatively crude methodologies to define genetic variation among viruses isolated from infants with congenital HCMV infections as well from viruses isolated from different sites in a single infant.2–6
Together, these studies have documented that individuals, including fetuses infected in utero, are not infected with a single strain of virus but a collection of genetically distinct viruses that in some cases approaches the genetic sequence diversity that closely resembles that found in RNA viruses.1 These findings coupled with a more complete understanding of the limited protection afforded by preexisting maternal immunity prior to conception in the prevention of superinfection/reinfection during pregnancy have challenged many long-held concepts of the natural history of congenital HCMV infections and argued that the natural history of this congenital infection must in some populations be redefined.
Case Definition and Incidence
The case definition of congenital HCMV infection that has been used in most large natural history studies of this infection has remained unchanged for decades and requires the isolation of replicating HCMV from the urine of an infant within the first 2 weeks of life.7 Since the early 2000s, the widespread use of polymerase chain reaction (PCR) in diagnostic laboratories has resulted in the increasing utilization of this technology in the diagnosis of congenital HCMV infections. Although PCR detection of HCMV is viewed by most investigators as equivalent to isolation of replicating virus, the possibility of false positives must be considered when samples such as saliva from breast-feeding infants are assayed. Positive PCR reactions in such cases can be confirmed by tissue culture isolation of virus from the urine of the infant or, alternatively, PCR analysis of urine from the infant.7 Using either tissue culture isolation of HCMV or PCR detection, investigators have reported that HCMV is the most common virus infection in the newborn infant that is acquired in utero, with reported rates between 0.5% and 2.0% in different populations throughout the world8 (Table 54-1). A large screening study of approximately 100,000 newborn infants in the United States that is nearing completion has reported that congenital HCMV infection occurs in about 0.5% of live births7 (Table 54-1).
Table 54-1 Incidence of Congenital Human Cytomegalovirus Infection in Different Geographic Locations
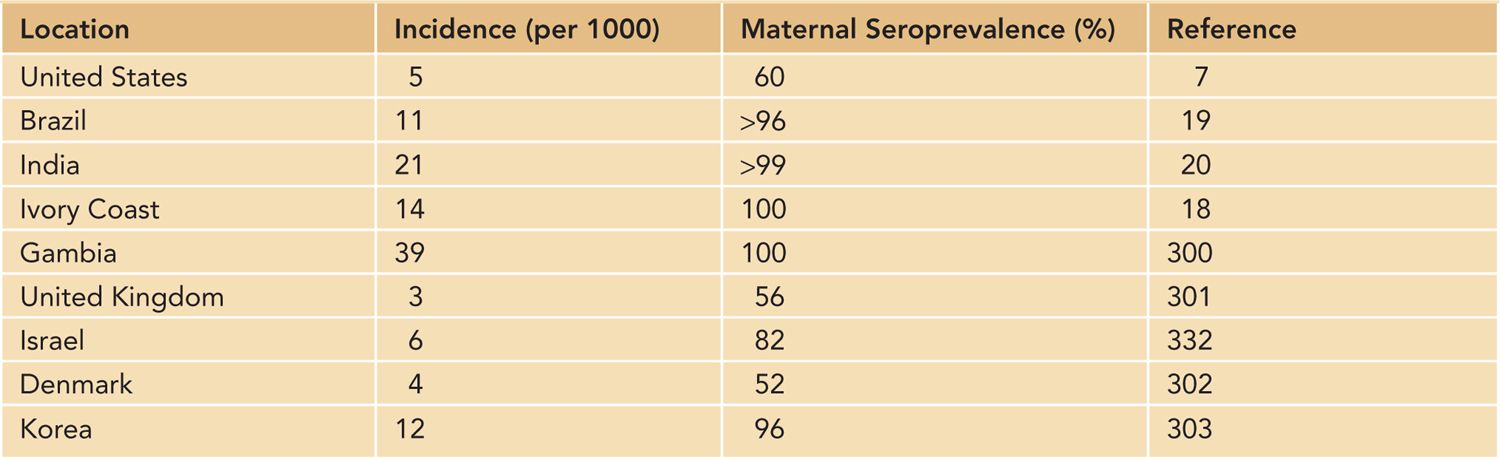
Previous studies from several institutions in the United States have reported rates of about 1%, primarily in urban, lower-socioeconomic populations.9 These rates are considerably higher than the rate of congenital HCMV infection in white middle- and upper-middle-class populations, a finding that suggests differences in both the risk of maternal infection and the nature of the maternal infection in women of different racial and socioeconomic groups.9–13 Congenital HCMV infections are common throughout most areas of the world, with rates between 1% and 5%, depending on the region of the world and the case definition of congenital HCMV infection8 (Table 54-1). However, in studies carried out using methodologies and case definitions consistent with currently accepted definitions, the rate of congenital HCMV infections in most populations appears to be approximately 1%.
A unique and incompletely understood feature of congenital HCMV infection is that it can occur in infants born to women who experienced a HCMV infection prior to pregnancy and have developed lasting serological immunity to the virus to HCMV, a characteristic that distinguishes congenital HCMV infections from other congenital infections, such as rubella and toxoplasmosis, but parallels the natural history of congenital syphilis.14,15 In fact, there is nearly a linear relationship between the rate of maternal HCMV seroprevalence and the rate of congenital HCMV infections, such that the highest rates of congenital HCMV infection can be found in maternal populations with near-universal HCMV seroreactivity.16 This finding is of great interest because maternal populations in most regions of the world, particularly the Southern Hemisphere, have very high rates of seroreactivity for HCMV, which in many populations approach 100%.17–21 These observations clearly demonstrate that existing maternal immunity does not prevent intrauterine HCMV infection and raises several issues that must be considered in the design and testing of vaccines that have been formulated for the prevention of congenital HCMV infections.
Clinical Features and Classification of Infection
The overwhelming majority of infants (90%) with congenital HCMV infections will exhibit no abnormal clinical findings in the perinatal period and will not be identified unless a newborn screening program is in place. These infants have been defined as asymptomatically infected for purposes of epidemiological studies. This classification also carries prognostic information because this population of infected infants has a lower overall incidence of neurologic sequelae associated with this intrauterine infection.11,22 The remaining 10% of infants with congenital HCMV infections will exhibit symptoms characteristic of perinatal infections but not diagnostic of congenital HCMV infection. These infants have been classified as symptomatically infected and have an increased risk of long-term sequelae.23,24 Physical findings can include hepatosplenomegaly; jaundice; microcephaly; petechial rashes; in rare cases cutaneous extramedullary hematopoiesis (blueberry muffin rash); neurological deficits, including seizures; and chorioretinitis23–25 (Table 54-2). Laboratory and imaging findings can include thrombocytopenia, direct hyperbilirubinemia with evidence of hepatitis, and abnormal findings in imaging studies of the brain of infected infants22,23 (Table 54-2).
Table 54-2 Findings in Infants With Symptomatic Congenital Human Cytomegalovirus Infection
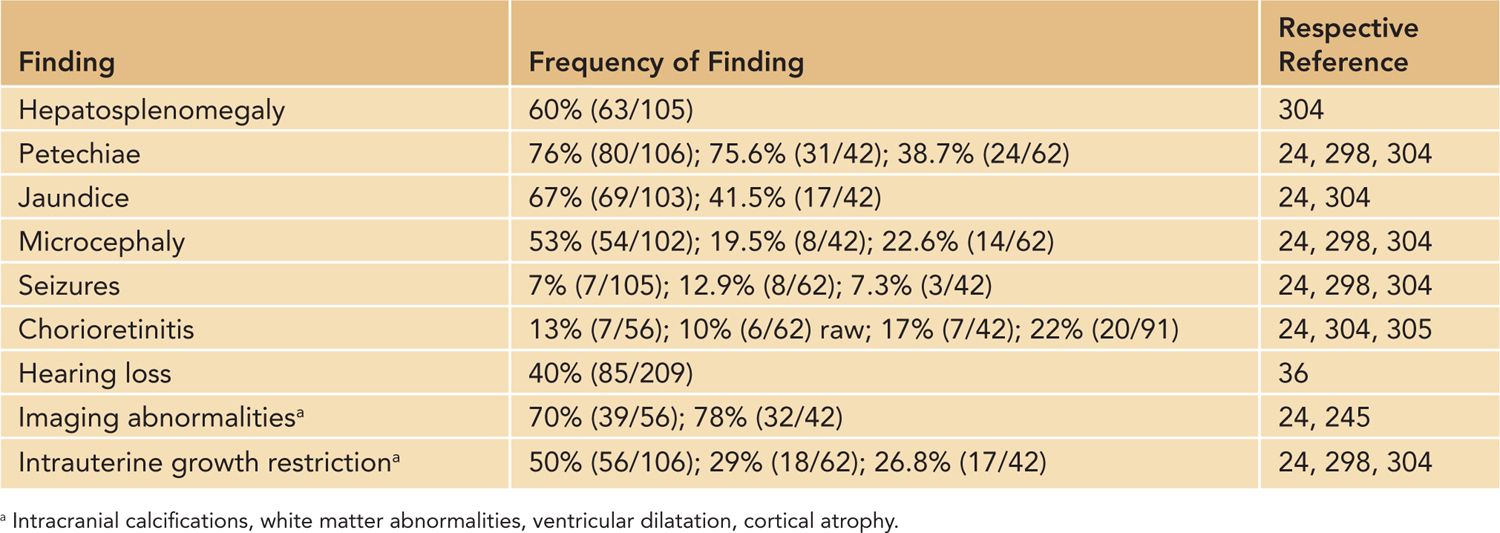
It is important to note that the initial case definitions of congenital CMV infection were based on clinical findings with additional laboratory studies prompted by clinical findings of petechiae and jaundice. Studies, including central nervous system (CNS) imaging, were not routinely carried out. Thus, more recent reports that include patients identified by studies other than clinical examination may not be completely comparable to the findings reported in the older literature. Severe infections have a mortality rate of 5%–11%, and long-term neurologic sequelae are frequent in infants with severe infections, reportedly occurring in up to 60% of symptomatically infected infants.23–27 Long-term sequelae are almost exclusively limited to the CNS, although poorly documented anecdotal reports of chronic liver disease were discussed in several reviews.
Several laboratory parameters have been identified that can further quantify the likelihood of long-term neurologic sequelae in a congenitally CMV-infected infant. Perhaps the most predictive of these but least well studied is the finding of encephalitis as defined by abnormalities in the cerebrospinal fluid (CSF) of infected infants.23,24,28,29 Similarly, imaging (ultrasound, computed tomography [CT], magnetic resonance imaging [MRI]) abnormalities of the CNS of infected infants, such as intracranial calcifications, including periventricular calcifications; evidence of migration deficits leading to lissencephaly and polymicrogyria; cerebellar hypoplasia; and white matter abnormalities all point to CNS involvement and a substantially increased risk of long-term neurologic deficits in the infected infant. These are discussed in the following sections.
The finding of chorioretinitis has been associated with an increased rate of neurodevelopmental abnormalities in infected infants and likely also reflects the involvement of the CNS in this infection.24,30 Attempts to utilize viral load measurements to identify infants at risk for neurodevelopmental sequelae, including hearing loss, have been disappointing when studied in screened populations of newborn infants primarily because of considerable variation in viral loads among individual infants.31–33 Overall, infants with symptomatic infections, particularly those with hepatosplenomegaly and thrombocytopenia, have increased levels of viral DNA in blood, urine, and other body fluids when compared to infants with asymptomatic infections, but these findings have not significantly refined the predictive value of the clinical findings in the individual patient.31,32 As an example, when blood viral load was investigated as a potential approach for early identification of infected infants with hearing loss, it appeared to lack sufficient positive predictive value for routine use in a screening program.33 Its negative predictive value was considered sufficient by some investigators, although many investigators believe such use in early infancy is unwarranted because up to 50% of congenitally infected infants with hearing loss will not exhibit evidence of hearing loss until after 6 months of age.34–38
Importance of Maternal Immunity in Transmission of CMV to the Fetus
Early studies of congenital HCMV infection reported that maternal immunity to HCMV played a critical role in limiting virus transmission to the fetus as well as in dramatically reducing severity of the ensuing intrauterine infection.11 Thus, maternal immunity to HCMV prior to pregnancy is widely believed to be a major determinant in decreasing the risk of transmission of virus to the fetus as well as decreasing the likelihood of a severe fetal infection in pregnant women infected with CMV. This concept remains a useful paradigm, but in the face of newer findings of reinfection of previously immune women with new strains of virus and the occurrence of symptomatic congenitally infected infants born to previously CMV-immune women, it cannot be viewed with the same level of certainty as in the past.
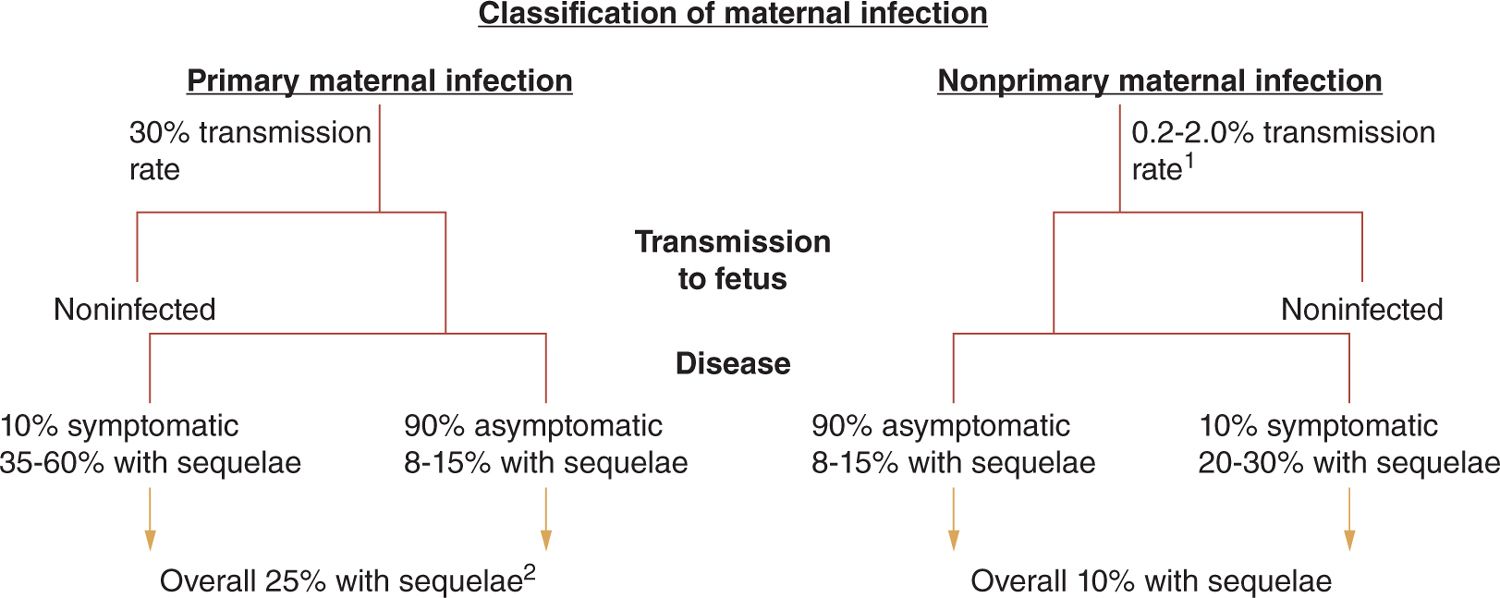
Maternal HCMV infections during pregnancy that occur in women without preconceptional seroimmunity to HCMV are classified as primary maternal infection, whereas those infections that occur during pregnancy in women with preconceptional immunity to HCMV are classified as nonprimary infections (Figure 54-1). Intrauterine transmission to the developing fetus occurs in about 30% of women undergoing primary infection during pregnancy as compared to somewhere between 1% and 2% of offspring born to women with nonprimary infection (Figure 54-1).11,19,26 However, it is important to note that the true rate of transmission in women undergoing nonprimary infection is unknown because it is unclear if all women with preconceptional seroimmunity to HCMV have the same risk (or mechanism) for transmission to their fetus or a similar rate of reinfection following exposure to a new strain of virus (see next section). Importantly, in most natural history studies, the ratio of congenital HCMV-infected infants born following nonprimary maternal infection as compared to primary maternal infection is at least 4:1 in many populations and likely higher in others, indicating that the congenitally infected infants delivered to women undergoing nonprimary infections represent the vast majority of infants with congenital HCMV infection.11,26 Thus, even if the incidence of symptomatic disease and long-term sequelae are less in infected infants following a nonprimary maternal infection as compared to that seen in infected offspring of mothers undergoing primary infection during pregnancy, infants infected following nonprimary maternal infection represent the major contributor to the disease burden associated with congenital HCMV infections.26
FIGURE 54-1 Natural history of congenital human cytomegalovirus (HCMV) infections in women without preconceptional serological immunity (primary) and those with preconceptional immunity and prior infection with HCMV (nonprimary). Intrauterine transmission rates and rates of disease and long-term sequelae are provided. 1Rate of transmission following nonprimary maternal infection is unknown but estimated from rates of congenital HCMV infections in immune maternal populations. This rate likely underestimates the true rate of transmission following maternal nonprimary infection. 2Rate of sequelae based on population of infants from screened cohort and referral population. As a result, the rate and severity of sequelae could represent an overestimate of true incidence.
Several studies have demonstrated that primary maternal infections that occur during the late first and early second trimester are more commonly associated with more severe manifestations in the infected newborn than those that occur in the third trimester, a finding consistent with the developmental status of the fetus at these different gestational ages.39–45 From studies of women undergoing primary infection, it also appears that the rate of transmission increases in the third trimester and occurs about twice as frequently as transmission in early gestation, a finding that parallels findings from studies of congenital toxoplasmosis, rubella, and syphilis.39,46,47
A caveat from these studies of congenital HCMV infections is that in most cases, the conclusions from these studies have been based on the gestational age of the fetus at the time of maternal seroconversion and not actual determination of intrauterine transmission in women undergoing seroconversion. Thus, it remains a possibility that the risk of intrauterine transmission (and disease) is related to the duration of exposure of the fetus to maternal infection and perhaps less of a function of a limited interval during the primary maternal infection. Such a distinction could be critical in intervention strategies, such as the use of passively transferred virus-neutralizing antibodies.
The virologic characteristics of primary maternal infection and virus transmission to the fetus have been detailed in a limited number of publications, whereas similar studies have not been accomplished in women with nonprimary infections during pregnancy. In women undergoing primary infection, the level of virus replication as measured by PCR analysis of the amount of viral DNA in peripheral blood failed to correlate with intrauterine transmission.42,43 Using maternal anti-HCMV antibody responses as indirect evidence of the level of virus replication, Alford et al. suggested that increased virus replication was associated with virus transmission.48 The limitations of this study, including the assumption that antibody responses in these women was a surrogate of virus replication, are readily apparent but do suggest that the virologic or immunologic characteristics of a maternal infection could influence the likelihood of transmission.
Finally, studies have demonstrated that women who deliver congenitally infected infants more commonly excrete virus in the postpartum period when compared to women who do not deliver an infected infant, regardless of the type of maternal HCMV infection.49 Sites of virus excretion include urine, cervical secretions, saliva, and breast milk.49,50
These results do not speak to a specific relationship between virus replication and intrauterine transmission of HCMV in some women but suggest the possibility that intrauterine transmission of HCMV could include the altered capacity of a subpopulation of women to control virus replication and dissemination. The variations in host-derived responses to HCMV in normal pregnant women as an explanation for differing rates of intrauterine transmission and the severity of the ensuing fetal infections have not been rigorously investigated.
Importance of Maternal Immunity and the Outcome of Fetal HCMV Infection
Studies of primary maternal CMV infections linked to congenital HCMV infections have failed to identify specific immunologic features of the maternal infection that could be directly related to intrauterine transmission of the virus. Studies in women undergoing primary infection during pregnancy failed to find a correlation between anti-HCMV CD4+ and CD8+ T-lymphocyte responses and virus transmission to the developing fetus.51 Studies that quantified maternal anti-HCMV antibody responses in relation to virus transmission to the fetus have demonstrated that women who transmit virus to their fetus often have higher levels of antiviral antibodies as compared to women who fail to transmit virus.52 When these studies quantified virus-neutralizing antibodies, a biologically more relevant antibody response, a correlation was found between the level of virus-neutralizing antibodies and protection from intrauterine transmission.52 Interestingly, this specific response was also directly correlated with the avidity of the antibodies reactive with the virus and a major viral envelope glycoprotein, gB, in that women with primary infection that failed to transmit virus to their offspring had, as a group, higher avidity antibodies.52
Finally, it is of interest to note that women in this study who transmitted virus to their offspring as a group had lower avidity antibodies and in some cases did not exhibit the expected avidity maturation with time that was observed in women who did not transmit virus to their offspring.52 This finding suggested a potential deficit in a maternal immune response associated with intrauterine transmission of HCMV.52 However, it is important to note that these older studies were done using only a single envelope protein, glycoprotein B (gB), and thus must be viewed as incomplete because of the more contemporary understanding of the importance of antibodies reactive with the HCMV pentameric gH/gL/Ul128–131 glycoprotein complex, a glycoprotein complex that determines epithelial cell tropism and one that is likely critical for the in vivo cellular tropism of HCMV.
The presence of preexisting maternal immunity has also been argued to be a major factor in the outcome of intrauterine HCMV infections.8,11,26,53,54 Early studies of congenital HCMV infections strongly supported an association between primary maternal infections and severe, clinically apparent congenital HCMV infections.11 This dogma continues and is firmly established in the literature even though early studies from Sweden and, more recently, studies from the United States, Sweden, and Brazil have demonstrated that the incidence of severe, clinically apparent newborn infections is similar between congenitally infected offspring of primary and nonprimary maternal infections.19,55–58 Some investigators have argued the most severely affected congenitally infected infants only result from intrauterine infection following a primary maternal infection and that infants with less-severe neurodevelopmental consequences of this intrauterine infection are more likely born to women with nonprimary infections.54,59,60 This difference in the views of investigators and findings that described severe infections with neurodevelopmental sequelae in infants born to women with nonprimary HCMV infections cannot be ascribed to misidentification of cases because in most reports these infected infants were identified by clinical findings that were consistent with standard case definitions.
Although this important aspect of the epidemiology of congenital HCMV infection continues to be contentious, ongoing screening studies that will enroll large numbers of infected infants should resolve this issue. It is also worth noting that in at least 1 of the larger series of patients in which the association between significant neurological damage with long-term sequelae and primary maternal infection was described contained a potential enrollment bias secondary to inclusion of infants from nonscreened populations who were identified secondary to clinically apparent infections at nonscreening sites. More thorough review of the original findings in this often-referenced study has raised the possibility that little difference in terms of neurodevelopmental sequelae exists between infants infected following primary and nonprimary maternal infections (S. Ross, 2012). Finally, in recent newborn screening studies of infants born to maternal populations with near-universal seroimmunity to HCMV prior to pregnancy, the rates of clinically apparent congenital infections and long-term sequelae, including hearing loss, appears similar to the rates reported in infants infected in utero following primary maternal infection.19,58
Maternal Infection and Congenital HCMV Infections: Sources of Maternal Infection
Human cytomegalovirus establishes a persistent infection in its host that is characterized by both reservoirs of latently infected CD34+ myeloid cells and a chronic productive infection in a number of different tissues (see following sections). During primary infection, infectious virus can be isolated from a number of sites, including the oropharynx, genital tract, urine, and blood. Virus excretion is prolonged in most acutely infected adults, and depending on the frequency and site of sampling, infectious virus can be recovered for months. In congenitally infected infants and in infants infected perinatally either during delivery or following exposure to infected breast milk, significant amounts of virus (102–3 infectious units/mL) can be detected in the urine and saliva for years.61–63 Thus, infants infected in utero and in the perinatal period represent an important reservoir of infectious virus that can serve to infect parents and other caretakers of HCMV-infected infants as well as other children, such as children in group care settings. CMV is readily transmitted during close and intimate contact and is considered a sexually transmitted infection (STI) (Table 54-3).
Table 54-3 Sources of Human Cytomegalovirus Infections
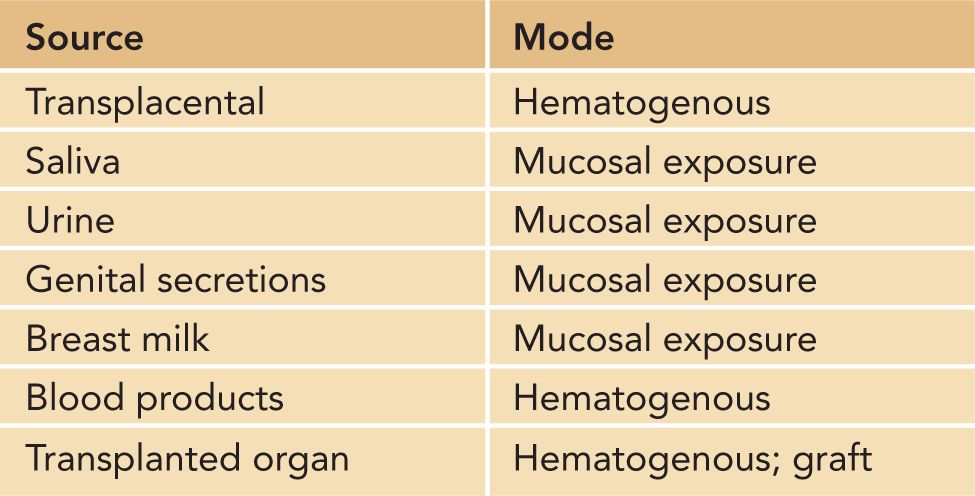
Epidemiological studies have consistently identified 2 sources of HCMV infection in pregnant women. Exposure to young children is thought to represent perhaps the most consistent risk factor for acquisition of HCMV that has been identified through epidemiological studies of community-acquired HCMV infections.64–68 Contact with young children in the home has been shown to represent a higher risk than does occupational exposure, suggesting that rigorous attention to hygienic precautions can limit virus acquisition by caretakers of young children.69–71 As noted, a second major route of HCMV infection is through sexual contact. High rates of HCMV infection have been documented in sexually active populations, including homosexual men, sex workers, and women attending STI clinics.72–76 Consistent with these observations, risk factors for the delivery of congenitally HCMV-infected infant include STIs such as gonorrhea and trichomonas.77
The relative contributions of exposure to young children and sexual transmission of HCMV to the incidence of maternal HCMV infection remain incompletely defined because of the difficulty of ascertainment of accurate exposure histories in most individuals, particularly sexual histories. However, several studies have documented the efficacy of counseling in preventing maternal infections with HCMV during pregnancy, with reductions in rates of maternal infections nearing 50%, an effect similar to the efficacy reported for a candidate HCMV vaccine.78–80 These studies have further reinforced the arguments of public health officials that an effective education program could have a significant impact on the incidence of HCMV infection in many populations.79
PATHOGENESIS
Introduction
A definitive understanding of the pathogenesis of CMV infections, in particular congenital CMV infections, has remained elusive for many reasons, including the genetic complexity of the virus, the paucity of informative animal models secondary to the strict species tropism of the HCMV, and ubiquity of infection with this virus in most populations in the world. Extensive efforts in studies of human infections associated with CMV have produced a large body of descriptive and correlative findings. Although these studies have led to greater understanding of the natural history of HCMV infections, definitive understanding of the mechanism of disease associated with HCMV infection is lacking. Clinical observations and studies in animal models have clearly documented the importance of the immune system in the control of the replication of HCMV in the infected host.
In no case was this more obvious than the spectrum of clinical infections observed in patients infected with human immunodeficiency virus (HIV).81 Yet, even in the immunocompetent normal host, a unique relationship between HCMV and the host allows persistence of the virus in the face of a robust immune response. In the normal, persistently infected host, a considerable proportion (up to 15%) of the peripheral CD8+ T-lymphocyte response can be directed at HCMV.82 Although a definitive explanation for the persistence of virus infection in the presence of such an overwhelming host immune response is not available, it has been argued that the myriad virus-encoded immune evasion functions could enable virus persistence in the normal host.83–87
A second and as yet incompletely defined characteristic of this virus that could contribute to its persistence is the extensive genomic diversity of the virus. Recent studies have demonstrated that HCMV circulates in the infected individual as a quasi-species with extreme genetic diversity.1 The relationship between this genetic diversity and virus persistence in the individual host remains unknown. Furthermore, the importance of the extensive genetic diversity of the virus to its transmission between hosts and its spread within populations is unknown. In almost all instances, attempts to define mechanisms of disease associated with HCMV infection have relied on relating disease with virus replication. It is unclear if a linear relationship between virus replication and disease exists in most cases, and without adequate definition of the host contribution to disease, thorough understanding of HCMV disease will likely remain elusive.
Sources of Virus and Routes of Transmission
The pathogenesis of CMV infections has been investigated in human subjects as well as in animal models. As discussed, infected individuals can shed HCMV from numerous body fluids, including saliva, urine, breast milk, and genital tract secretions. HCMV DNA has been identified in hepatocytes, renal epithelium, and pancreatic acinar cells from adults experiencing sudden demise from trauma or cardiac arrest.88,89 HCMV DNA has also been identified in epithelial tissues of salivary glands, breast, and prostate tissue from normal asymptomatic hosts, and infectious virus has been recovered from saliva, urine, breast milk, and prostatic secretions from healthy hosts, suggesting that the epithelium in these sites can serve as a reservoir for the production of infectious virus that is released intermittently from asymptomatically infected individuals.8
It then becomes obvious how secretions/excretions from these sites can readily promote the spread of HCMV throughout populations. Young children excrete large quantities of infectious virus asymptomatically in saliva and urine, promoting person-to-person spread by close contact among young children and their caregivers. Nonepithelial reservoirs include mononuclear cells and endothelial cells, which more often serve as sources of virus in noncommunity infections, such as those acquired through blood transfusions or organ allograft transplantation.
In medical settings, in addition to transmission through conventional pathways, alternative modes of HCMV transmission include blood transfusion and transplantation of infected organs or stem cells. Because monocytes may serve as reservoirs of latent infection, use of irradiated blood and blood filtered to deplete leukocytes decreases the likelihood of virus transmission via blood transfusion.90–95 However, cell-free virus may also exist in human blood products; thus, even though the risk of HCMV transmission through transfusion of leukocyte-reduced blood products remains low, it cannot completely be excluded by current practices.95 The potential risk of blood transfusion-acquired HCMV infection in neonates has been recognized for over 3 decades.96–98 Early studies demonstrated that term infants born to HCMV-seronegative women and premature infants were at risk for HCMV infection and disease from transfusion of blood from HCMV-seropositive donors.97 HCMV can also be transmitted via transplantation of an infected solid organ or via stem cell transplantation. Reservoirs of infection are likely to include epithelial and endothelial cell reservoirs in solid organs and CD34+ myeloid progenitor cells in stem cell transplantation.99–102 To date, strategies have not been devised or tested that could prevent reactivation from infected donor tissues, but the use of antiviral agents as prophylaxis against viral dissemination have significantly reduced the incidence of HCMV disease in seronegative transplant recipients receiving infected donor organs.103–105 This last clinical observation is consistent with reactivation of latent infection as a significant source of HCMV infection in allograft recipients.
Transmission in normal individuals in a community setting is thought to occur via contact with infected secretions in the oropharynx or following sexual contact and exposure to genital secretions. HCMV envelope glycoproteins appear to determine attachment and entry of infectious virions into susceptible cells. Thus far, glycoproteins B (gB), H (gH), and L (gL) have been shown to be essential for infectivity in human fibroblasts under laboratory conditions, whereas the pentameric envelope glycoprotein complex of HCMV UL 128–131 (consisting of UL128, 130, 131a, gH, and gL) is required for entry into epithelial, endothelial, and mononuclear cells in vitro.106–108 The role of gO, a component of gH/gL, in entry remains incompletely defined because of conflicting findings from a number of laboratories.109–113 Entry in fibroblasts is pH independent and has been reported to occur by virion envelope fusion at the cell membrane, whereas entry in epithelial cells utilizes a receptor-mediated endocytosis followed by a pH-dependent fusion event.107,114–116 Recent findings have suggested that HCMV enters fibroblasts through an actin-dependent step that resembles macropinocytosis that has been described in phagocytic cells. The cellular receptor(s) mediating entry of HCMV remains undefined, although low-affinity interactions with cell surface proteoglycans have been shown to contribute to the initial attachment of the particle.114
Following entry into the host cell, a number of intracellular signaling events have been documented.116–120 In most cases, the contributions of signaling through these pathways to the biology of the virus infection have yet to be fully determined. Similarly, intercellular events in these proximally infected, presumably epithelial, cells remain uncharacterized; however, based on animal models of cytomegalovirus infection, virus most likely passes through the mucosal surfaces and replicates in regional lymph nodes, resulting in primary viremia, and is followed by secondary replication in the liver and spleen. Thereafter, a secondary viremia develops, which is asymptomatic in most individuals but occasionally produces clinically observable symptoms, including fever, malaise, pharyngitis, and lymphadenopathy. Recent studies in a murine model of CMV dissemination in immunocompetent animals suggested that dissemination likely occurs during the primary viremia, and the seeding of end organs is less dependent on the amplification of virus titer in organs such as the liver and secondary viremia.121,122
Although these findings potentially could explain the lack of a linear relationship between viral loads in blood/plasma and disease in individual patients at risk for end-organ disease, they also fail to account for observations made in immunocompromised patients, such as transplant recipients and HIV-infected hosts who develop end-organ disease after prolonged periods of viremia.123–125 Perhaps an explanation for the apparent differences in the pathogenesis of CMV dissemination is related to primarily a finite period of cell-associated viremia in the immunocompetent host vs a prolonged combination of cell-free and cell-associated viremia in the immunocompromised host. Viremia appears to be limited by host immune responses that control viral replication in diseased organs and curtail ongoing dissemination. In HIV-infected hosts, this control has been most convincingly associated with HCMV-specific CD4+ T-lymphocyte responses.126,127
Cells of monocyte/macrophage lineage can support productive infection.99,128–131 In vitro and animal studies have suggested that recruitment of infected mononuclear cells to sites of inflammation can promote transfer of virus to local end organs, providing a mechanism for dissemination of virus to distant sites by hematogenous cell-associated spread in humans.101,132,133 In experimental mouse models, investigators have demonstrated that expression of virus-encoded functions that share sequence similarities with chemokines but do not signal through known G-protein coupled receptors can increase recruitment of myeloid cells into sites of initial virus replication and therefore increase the efficiency of dissemination from sites of primary replication.132 Viral gene products of HCMV have been characterized to share sequence and functional homology with human interleukin (IL) 8, a cytokine that has been associated with recruitment of myeloid cells.134 Alternatively, during primary infection, cell-free virus may circulate in blood and directly infect susceptible cells in almost every organ.
In the normal host, HCMV likely establishes a persistent infection, replicating at low levels and entering what has been termed a quiescent state or, like other herpesviruses, may enter true latency. During latency, viral genomes within infected cells exhibit restricted viral gene expression without the production of late gene products or infectious virions. Reservoirs of persistence and latency in humans have been proposed based on in vitro analysis of human cell types in cell culture and from animal models, in particular CMV infection in mice (murine CMV, MCMV). Sites of latency are thought to include monocytes, endothelial cells, epithelial cells of the lung, spleen, kidney, prostate, cervix, and salivary glands. Several in vitro studies have demonstrated latent infection of CD34+ myeloid progenitor cells, and it is believed that these cells could be responsible for dissemination of HCMV following mobilization of this cell population for stem cell transplantation and reactivation of HCMV replication.101 The nature of latency, including establishment, maintenance, and reactivation of latent infection in CD34+ cells, remains poorly understood and has been investigated primarily using in vitro systems. More recently, elegant systems utilizing humanized mice models to study HCMV infection in vivo have provided a platform that could permit a more definitive understanding of this complex virus-host interaction.
Perinatal HCMV Infection: Breast Milk Transmission
Human cytomegalovirus has been demonstrated to be present in breast milk of lactating women, primarily as cell-free virus.135–137 Asymptomatic infection of term neonates via breast feeding has been demonstrated in approximately 60% of breast-fed infants of HCMV-seropositive women and almost certainly represents the most common mode of transmission of this virus in the world.50,136 Although HCMV can be transmitted to premature infants via oral ingestion of infected breast milk, postnatal infection by this route has not been reported to be associated with CNS damage or other adverse late outcomes that are seen following congenital infection.138 The pathogenesis underlying these observations has not been characterized but may be caused by differences in the mode of transmission (oral vs hematogenous), host maturity at time of infection, or perhaps potential differences in timing of damaging congenital infection in utero (first and second trimester) compared to infection of an infant at the age of viability. The lack of reported CNS disease in premature infants acquiring CMV following breast feeding must be interpreted in the context that less than 10% of congenitally infected infants exhibit CNS involvement, raising the possibility that results from studies of the role of breast feeding in premature infants could have been underpowered to document the true incidence of long-term sequelae following breast milk transmission of HCMV. Of considerable clinical importance, preterm infants can develop severe acute CMV infection, including pneumonitis, colitis, and viral sepsis.139–142
Intrauterine Transmission of HCMV: Placenta Infection
In congenital infection, the virus must pass through the placenta to enter the fetus, presumably by hematogenous spread to the placenta. Analysis by in situ DNA hybridization, PCR, and immunohistochemistry of placentas from women who delivered HCMV-infected infants have demonstrated evidence of HCMV infection in epithelial cells, cytotrophoblasts, syncytiotrophoblasts, placental fibroblasts, and endothelial cells.143–150 In placentas with chorioamnionitis, HCMV DNA has been detected in maternal macrophages.149 Immunohistochemical staining for HCMV proteins showed the presence of HCMV gB in fetal neutrophils, macrophages, and dendritic cells in the villus core.148,151 In vitro studies suggested that cytotrophoblasts and syncytiotrophoblast cells are permissive for HCMV infection.143–145,152,153 Interestingly, placental cells infected with HCMV in vitro can produce proinflammatory cytokines IL-6 and IL-8, and more recently, a proinflammatory signature of cytokines was found in the amniotic fluid of fetuses ultimately shown to be infected with HCMV in utero.154,155 However, these in vitro studies have not yet been extended to studies of HCMV-infected placental tissues in vivo, and mechanisms of transmission and protection from intrauterine transmission remain undefined.
Studies of placentas derived from women delivering infected and noninfected newborn infants have suggested that antiviral antibodies could play a major role in limiting virus transmission to the fetus and could account for the protective functions of passively acquired immunoglobulins.54,156,157 In vitro infection of human umbilical vein endothelial cells showed induction of αVβ6-integrin-mediated activation of transforming growth factor β, a potent fibrogenic cytokine, as well as collagen synthesis, suggesting an effect of HCMV infection on extracellular matrix remodeling in the infected placenta.158
More recently, investigators have begun to suggest that HCMV-induced placental dysfunction could be an important comorbid event during maternal HCMV infection during pregnancy that could account for several of the more significant findings in infants with symptomatic congenital HCMV infection. These include disruption of normal placental architecture, a vasculopathy, and as noted, the creation of an inflammatory mileu.54,146,159 This topic is rapidly evolving and extremely important for a more complete understanding of the pathogenesis of congenital HCMV infections. For those interested, several excellent reviews of the interactions between placental infection and HCMV are available.148,160
Establishment of animal models for congenital CMV infection have been hampered by lack of transplacental passage of CMVs in murine models, thought to be related to the number of cell layers at the maternal-fetal interface in these animal placentas.161,162 The guinea pig placenta, like the human placenta, has only 1 cell layer at the maternal-fetal interface; thus, this model has been used as a model for congenital CMV infection to examine correlates of protective maternal immunity against damaging congenital disease.163–171 However, the guinea pig model has some limitations, including a high incidence of severe disease and mortality among infected pups secondary to the extremely fulminant nature of this model of congenital infection. From reports that have described the guinea pig model of congenital HCMV infection, it is clear that congenital infection takes place, but the data are less compelling that disease in congenitally infected guinea pigs is secondary to virus infection of the developing guinea pig and could be secondary to significant placental dysfunction in the infected dam, as was suggested by studies in humans.172
Immune Control of HCMV Infection: Intrinsic and Innate Responses
The initial control of acute HCMV infection in the normal host is initiated by what have been described as intrinsic and extrinsic innate responses that include responses of the infected cell and innate immune responses, including the antiviral activity of natural killer (NK) cells and cells of the myeloid lineage, including tissue macrophages (Table 54-4). Cellular intrinsic responses have been identified primarily through studies that have described the capacity of virus-encoded gene products to modulate these responses in infected cells. An example of a viral function that limits cellular intrinsic responses is the virion tegument protein, pp71, which has been demonstrated to function as an inhibitor of the cellular protein Daxx, a nuclear protein that targets exogenous nucleic acids of degradation173,174 (Table 54-4).
Table 54-4 Mechanisms of Resistance to Human Cytomegalovirus Replication
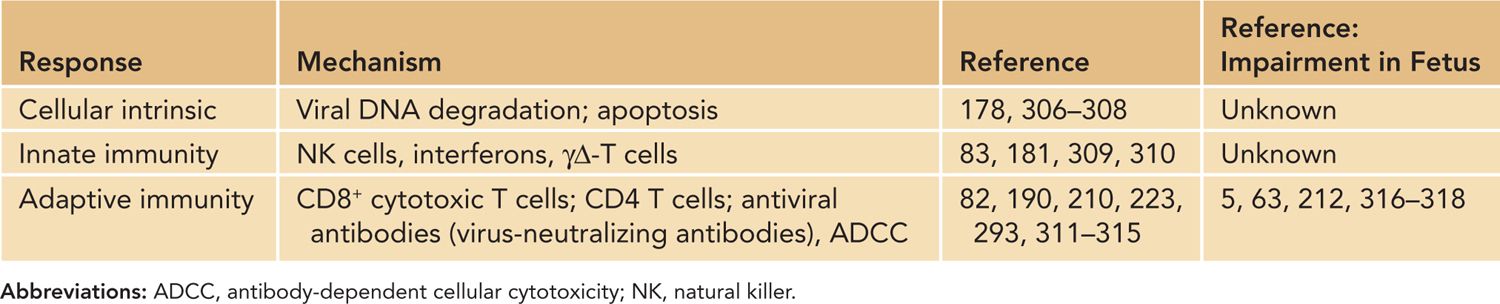
Additional viral functions have been described that limit intrinsic responses, including the formation of promyelocytic leukemia (PML) bodies, a host response that sequesters nucleic acids in the nucleus and limits transcription from the HCMV genome, induction of interferon (IFN) responses, blocks programmed cell death through either apoptosis and necrosis, and increases the autophagic activity in infected cells.174–178 Together, these viral functions are thought to enhance the capacity of the virus to establish infection, initiate viral gene expression, and permit productive infection in target cells. In the case of HCMV, the virus encodes an IL-10-like molecule that does not share structural or antigenic relatedness to the host IL-10 yet retains IL-10 function.179 The production of the viral IL-10 early in infection has been postulated to inhibit the differentiation of myeloid cells into dendritic cells in the local sites of infection and potentially block early recognition of virus-infected cells by host responses.180 The importance of extrinsic cellular responses such as NK cells in early control of herpesvirus infections has been shown in case reports describing severe herpesvirus infections in patients lacking NK cell function and a large number of studies in murine models.181–184
Additional evidence of the critical role of natural immunity in the control of CMV infections is perhaps best illustrated by the détente that appears to have been reached by the virus and host antiviral responses in normal individuals.87 The genomes of both HCMV and MCMV encode numerous open reading frames (ORFs) that express proteins that can modulate surface host protein expression to evade host NK recognition of virus-infected cells, including downmodulation of major histocompatibility complex (MHC) class I, production of decoy MHC class I homologs, and downregulation of surface expression of ligands for the activating NK group 2 receptor (such as MICA/B, MULT-1, RAE-1, and H60).185 The evasion of specific host responses following expression of virus-encoded genes illustrates a viral strategy for persistence but not virulence in the normal host. It is likely that such viral immunoevasins developed during the coevolution of CMVs with their natural hosts.87
Immune Control of HCMV Infection: T-Lymphocyte Responses
Long-term control of HCMV infection is achieved by an adaptive immune response to HCMV, primarily through the activities of HCMV-specific T-lymphocyte responses. Expansion of cytotoxic effector CD8+ T lymphocytes terminates productive primary infection in animal models of human disease.183,186–188 Interestingly, in these models CD4+ T cells were also capable of rescuing infected immunodeficient mice from lethal infection.183 Establishment of HCMV-specific memory T cells limits viral shedding and may limit reactivation from latency.189 Similar findings have been approximated in human studies. Adoptive transfer of HCMV-specific T cells in patients after hematopoietic stem cell transplantation was pioneered as a potential strategy to prevent HCMV disease in these immunodeficient patients but has not evolved into standard therapy for high-risk patients.190,191 Interestingly, in these pivotal studies, reconstitution of HCMV-specific CD8+ CTL (cytotoxic lymphocyte) responses were associated with early control of virus replication, but long-term control of HCMV infection required the reconstitution of HCMV-specific CD4+ T-lymphocyte responses.190
Perhaps a unique aspect of the immunobiology of CMV infections is the magnitude and breadth of the T-lymphocyte response to this virus. Early studies readily detected both HCMV-specific CD4+ and CD8+ T-lymphocyte responses to crude antigens and virus-infected cells. As technology advanced, the distinctive characteristics of this response were cataloged. Once assays for intracellular cytokine production following antigen stimulation of T lymphocytes from immune individuals became routinely employed for measurement of T-lymphocyte responses to HCMV, many laboratories reported unsuspected features of this response.
As noted previously, some studies included the finding that, in healthy immunocompetent individuals, up to 10%–20% of peripheral blood T lymphocytes were specific for HCMV.82 This finding was mirrored in mice infected with MCMV, and the phenomenon of memory inflation of the CD8+ T-lymphocyte response became a distinctive characteristic of the CD8+ T-lymphocyte response to CMVs.82,192 The commitment of such a significant proportion of the host’s CD8+ T-lymphocyte response to 1 agent has been argued by some investigators as an explanation for immune senescence in older individuals leading to decreasing T-lymphocyte function in the elderly.192–194
A second finding that was somewhat unexpected was that normal immunocompetent individuals infected with HCMV generated T-lymphocyte responses to a large number of viral-encoded proteins, including a large number of virion structural proteins.82 This finding provided support for the argument that HCMV likely persists in the infected host as a chronic productive infection with the expression of late gene products that can induce a measurable T-lymphocyte response.82
Finally, the availability of fluorochrome-conjugated tetramers (peptide epitopes folded with MHC class I molecules) have enabled investigators to precisely interrogate the viral antigen-specific CD8+ T-lymphocyte response in a variety of patient populations in real time. This approach has allowed the enumeration of CD8+ T-lymphocyte responses during acute, chronic, and reactivated infections and has provided prognostic information on patient outcome in patients at risk for severe infections with HCMV.195,196 Together, these technological advances have advanced our understanding of the immunology of HCMV infections as well as providing a solid foundation for future studies of the role of T-cell immunity in the natural history of HCMV infections.
Adaptive Immune Responses: Antiviral Antibodies
Infection with HCMV induces a diverse and readily detectable antibody response that also includes the production of rheumatoid factor-like antibodies. Virus-specific antibodies are made shortly after infection and include an initial IgM response followed quickly by IgG antibodies. Rarely can an individual be identified with only IgM anti-HCMV antibodies and undetectable levels of IgG antibodies reactive with HCMV, thus making it difficult to accurately ascertain seroconversion with patient specimens provided during the early period after infection. However, several laboratories have demonstrated that the IgG antibody response undergoes an avidity maturation that can be easily measured in antibody-binding assays.197 The avidity maturation of the IgG anti-HCMV antibody response forms the basis of diagnostic assays widely used in Europe to assess the duration of a serologically detected HCMV infection. The utility of such assays when combined with screening for HCMV-specific IgM antibodies has permitted new insight into the natural history of maternal infections during pregnancy.
A broad repertoire of antiviral antibodies is produced against a large number of structural and nonstructural proteins produced within infected cells. In terms of antiviral antibodies that are thought to function in protective responses, most studies have indicated that antibodies directed at the envelope proteins of the virus are most consistently associated with protection in vivo. A detailed explanation of the antienvelope protein antibody response is likely beyond the scope of this discussion, but suffice it to say that the envelope of HCMV is extraordinarily complex and antibodies against most of the major protein components of the virion envelope have been shown to exhibit some virus-neutralizing activity assayed in vitro, a surrogate for in vivo protection (Table 54-4). In some cases, this activity appears to be limited to virus infection of human fibroblast cells, whereas in other cases antiviral antibodies against specific virion envelope proteins are more effective in neutralizing virus infection of epithelial cells.198–207
It is unclear at this time whether a single-antibody specificity is critical for protective responses in vivo, but it seems unlikely in view of the large numbers of antienvelope antibodies produced during infection. Data from a large number of studies have suggested that antiviral antibodies with virus-neutralizing activity as measured in vitro can modify disease associated with HCMV infections in a number of populations, including solid-organ transplant recipients, HIV-infected individuals, and in the infected newborn.52,54,63,208–212 In many of these studies, protection from disease and not infection was achieved by the passive transfer of antiviral antibodies prepared from HCMV-immune donors.54,210–212 Similarly, support for a protective role of antiviral antibodies has been provided from studies in a number of animal model systems.167,213–215 However, it must also be noted that a detailed understanding of the mechanism of antiviral antibody protection in humans infected with HCMV and in most animal models is lacking. In addition to virus neutralization, data from animal model systems have suggested that cellular effector functions such as antibody-dependent cellular cytotoxicity (ADCC) participate in these protective responses.216
An important aspect of the proposed protective activities of antiviral antibodies, in particular virus-neutralizing antibodies, is that the virus-neutralizing antibody responses have been suggested as possible surrogates for the protective activity of candidate prophylactic vaccines. In fact, 1 of the most extensively tested vaccine candidates is a glycoprotein (gB) found in the envelope of the virus and a well-described target of virus-neutralizing antibodies.217,218 Early studies argued for an association between the amount of antibodies reactive with gB and outcome of infection. Moreover, recently a small vaccine trial in renal transplant recipients utilizing an adjuvanted gB vaccine provided provocative data that was interpreted as evidence for a role in virus-neutralizing antibodies in limiting disease in these immunocompromised hosts.219
Last, an initial study that was uncontrolled and unfortunately has been followed with several observational studies that were also flawed either because of the lack of controls or biased enrollment have argued that congenital HCMV and its sequelae can be prevented by the administration of pooled human intravenous immunoglobulin (IVIG) in women undergoing primary HCMV infection during pregnancy (see following sections).212 The quality of these studies is such that most investigators believe the data impossible to interpret; however, the possibility that such treatment could be efficacious has prompted additional trials using IVIG following documentation of primary maternal infections during pregnancy. The results of these studies should be available sometime during the next several years. Interestingly, a well-designed and controlled trial using the same preparation of the IVIG as was used in the initial trial failed to show any protection from fetal infection.330
From these data and studies in animal models, several pharmaceutical programs have initiated the development of systems that will permit production of large quantities of either human or humanized monoclonal antibodies that have potent in vitro neutralizing activities. It is anticipated that these antibody preparations will be used in either prophylactic or treatment protocols to limit disease secondary to HCMV infection in a number of different clinical settings, ranging from transplant recipients to fetuses exposed to HCMV in utero.
Immune Responses in the Congenitally Infected Infant
Immune responses in congenital CMV infection have been studied in a limited number of patients. In 8 neonates with congenital CMV infection, determination of postnatal CD8+ T-cell responses revealed higher proportions of activated CD8+ T cells which expressed surface markers including HLA-DR and CD95 but lower levels of CD62L, suggesting antigen exposure.220 In addition, these investigators demonstrated that these CD8+ T lymphocytes expressed differentiation markers, including CD45RO but low levels of CD28 and CD27, suggesting these cells represented an effector-memory population, a finding that contrasted to CD8+ T lymphocytes isolated from uninfected newborns.220
Spectratyping analysis suggested oligoclonal expansion of antigen-experienced clones, compared to the naïve phenotype of CD8+ cells from control uninfected newborns.220 CD8+ T cells from infants with congenital HCMV infection also demonstrated cytotoxic and cytokine production when compared to cells from uninfected neonates. These findings suggested that damaging intrauterine HCMV infection associated with congenital HCMV infection may not be prevented by establishment of antiviral CD8+ T-lymphocyte responses in the fetus.220
Similar findings have been described in a study of 16 fetuses, although these authors suggested that overall the responses exhibited a slight decrease in IFN-γ responses following in vitro stimulation.221 In a later study, Gibson and colleagues also documented HCMV-specific CD8+ T-lymphocyte responses in the first day of life in congenitally infected infants and at later time points in young infants, indicating that young infants were capable of generating these adaptive responses.222 In addition, these authors correlated the presence of these responses in infants with a decrease in the viral load in peripheral blood, suggesting a role of this response in control of HCMV replication.222 Altogether, these studies have argued that CD8+ T cells have the capacity to expand and differentiate in response to intrauterine viral infection such that by the time of birth, circulating CD8+ T cells can exhibit a mature and antigen-specific phenotype.
In contrast, other investigators have argued that adaptive immune responses, specifically CD4+ T-lymphocyte responses to HCMV, are depressed in HCMV-infected infants compared to adults.223,224 Similar results that described decreased CD4+ T-lymphocyte responses to HCMV in HCMV-infected infants were reported following studies carried out nearly 3 decades ago.63,225,226 However, advances in the analysis of HCMV-specific T-lymphocyte responses that were described previously have made the extrapolation of results from these early studies to current findings somewhat difficult. Thus, the relationship between results from these early studies and those from more recent studies to the natural history of congenital HCMV infections remains undefined. However, it is interesting that a more recent study suggested that neonatal dendritic cells exhibit deficient IL-12 and IFN-γ production following exposure to HCMV.227 This finding could potentially explain the previously described deficits in CD4+ T-lymphocyte responses in infected infants.
The study of fetal and neonatal adaptive immune responses to infectious agents is an active area of research, and HCMV-infected infants offer an ideal opportunity to further define these responses in young infants. For more details, those are referred to recent reviews in the immunobiology of CMV infection in the fetus and neonate.228
DISEASES IN INFANTS WITH CONGENITAL HCMV INFECTION
Introduction
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree