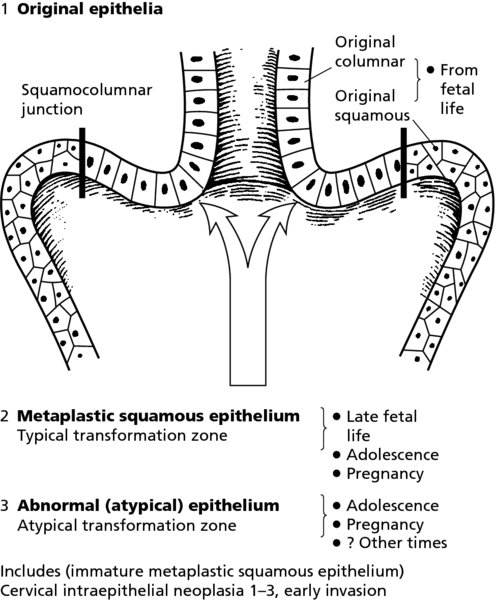
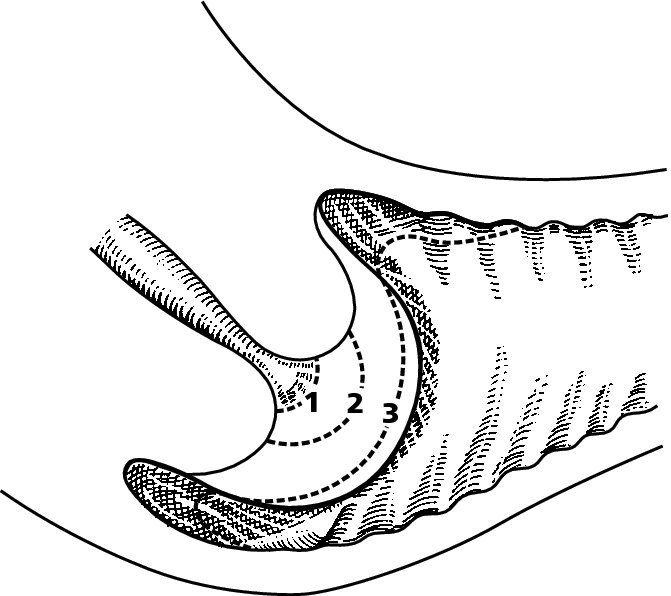
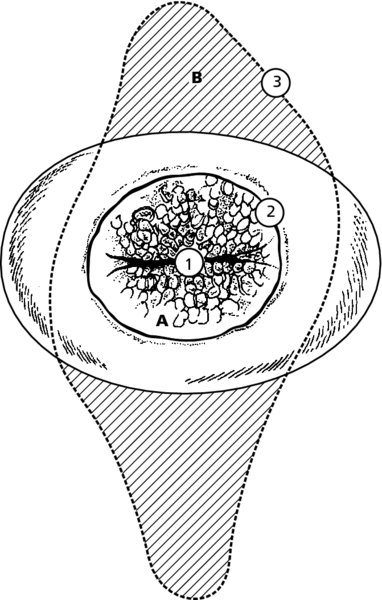
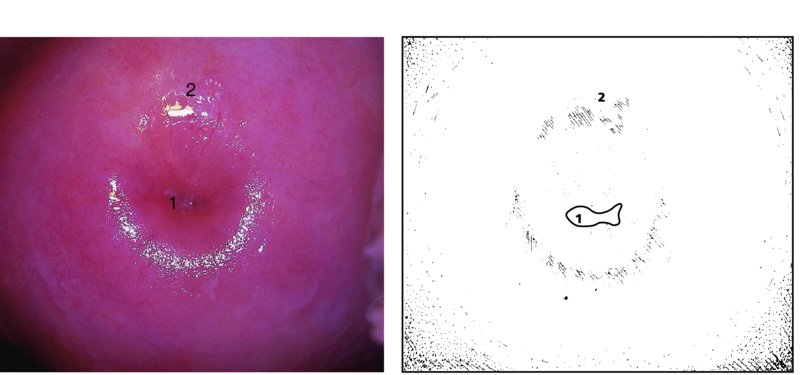
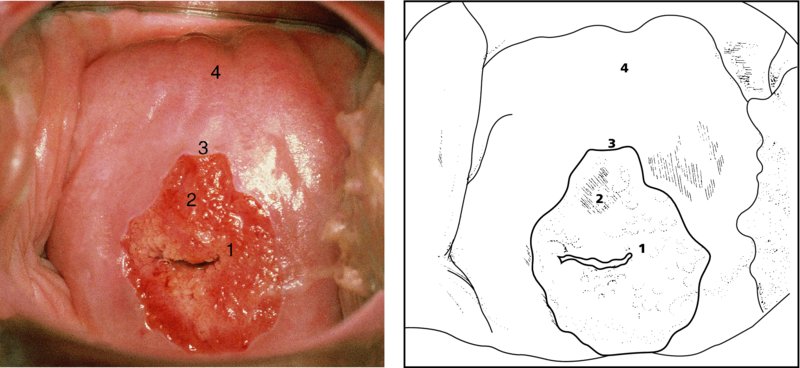
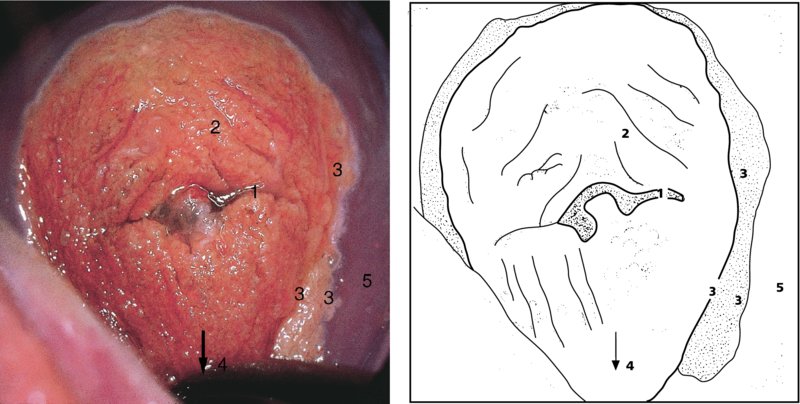
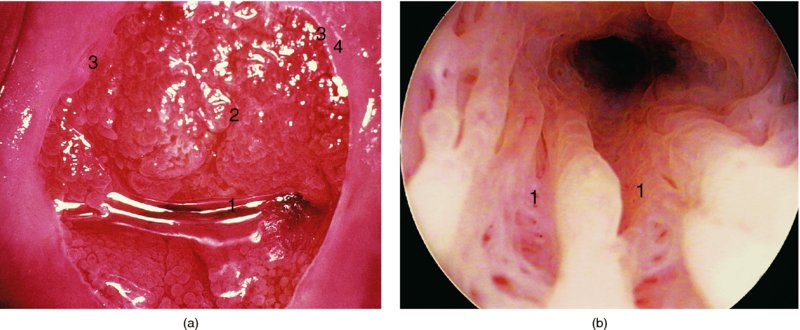
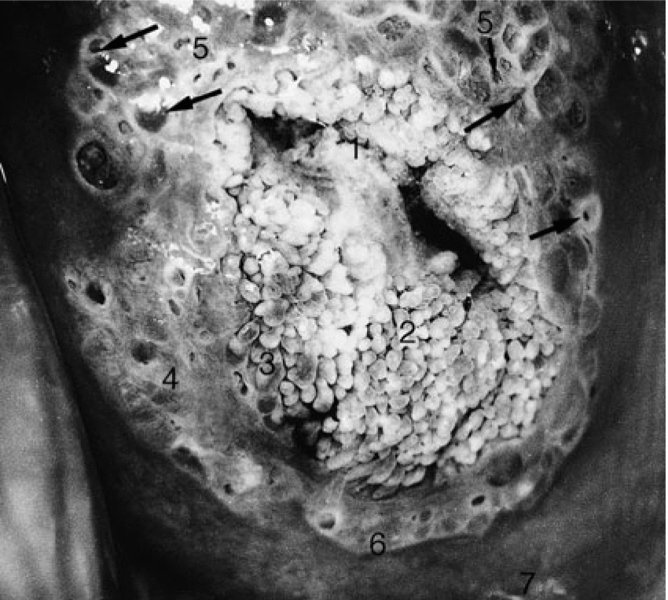
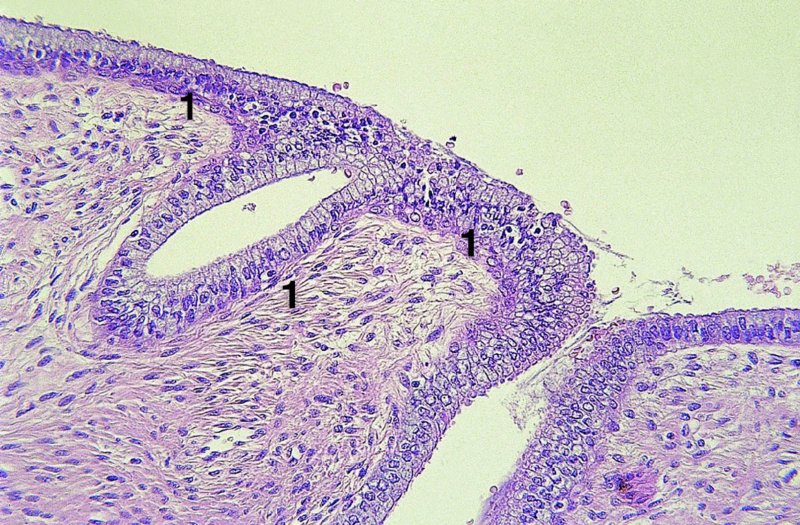
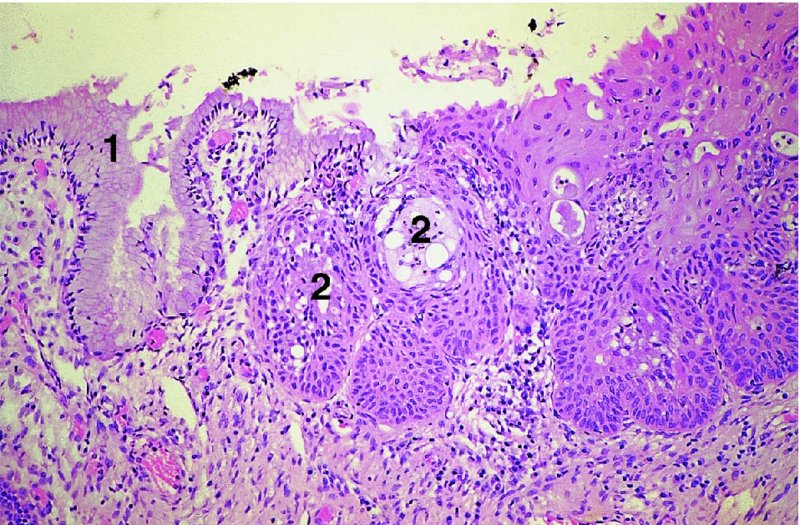
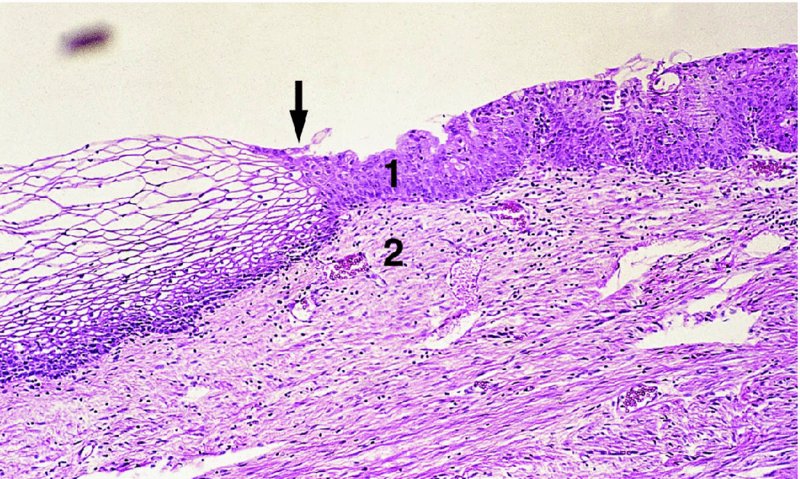
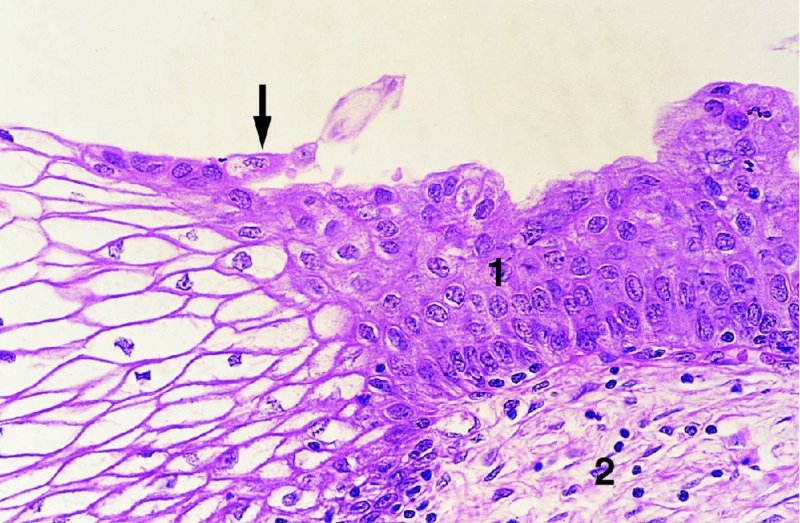
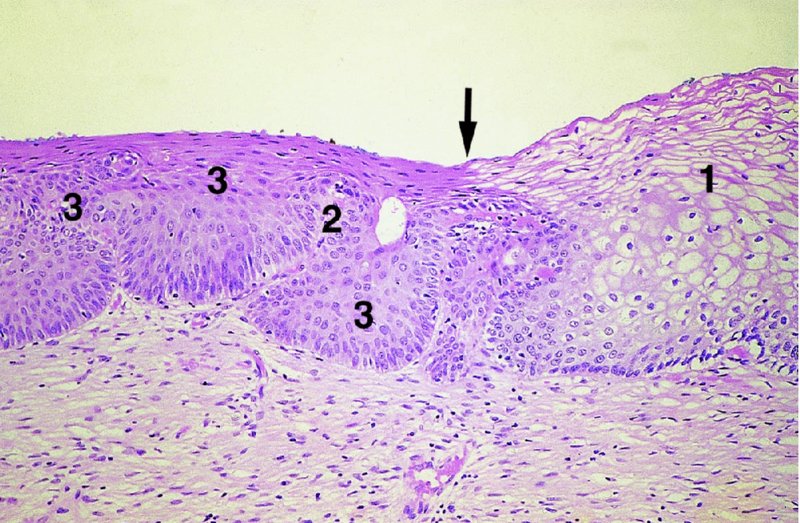
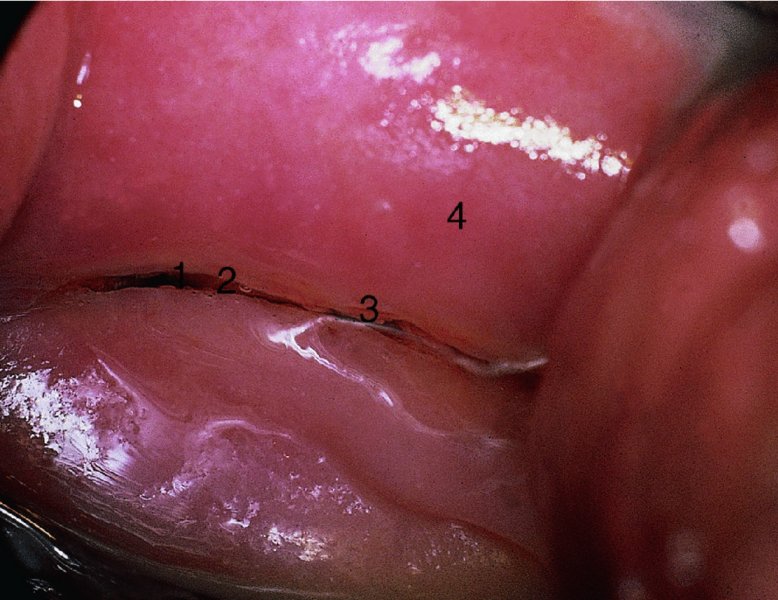
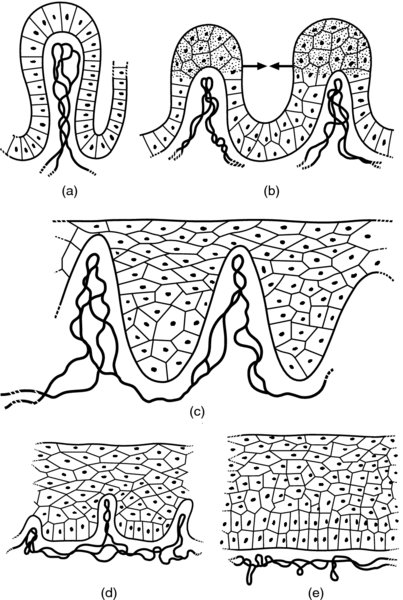
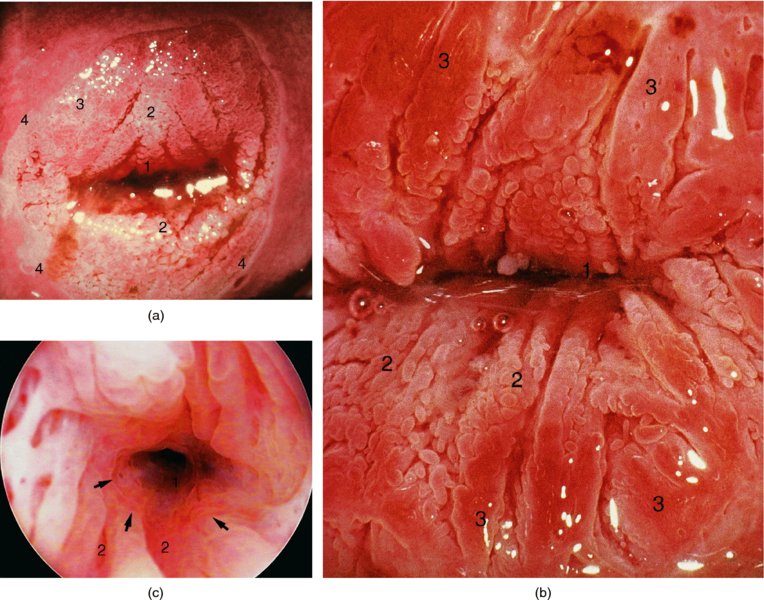
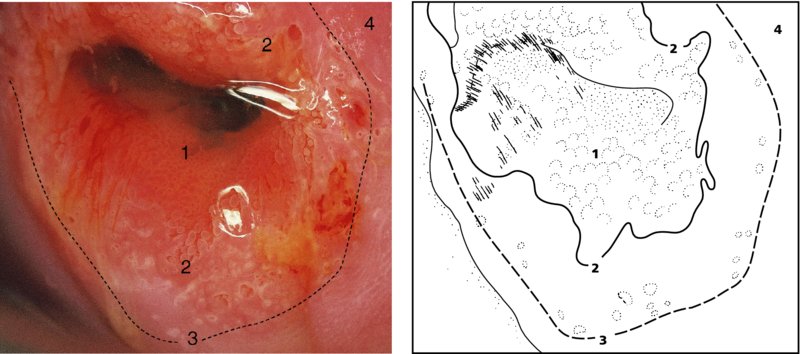
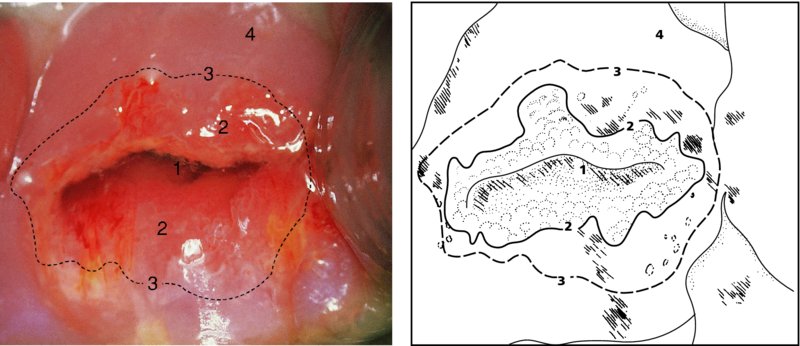
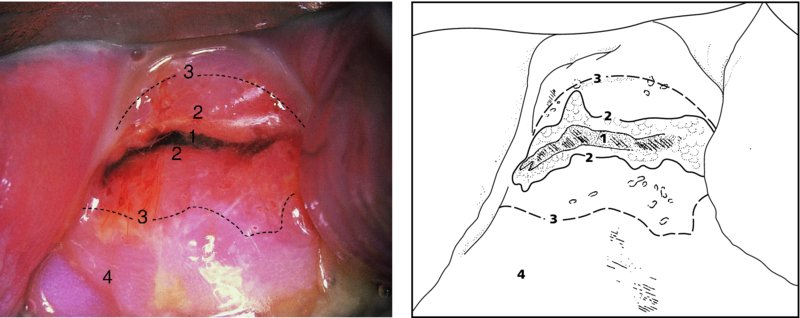
CHAPTER 4 Together with cytology, colposcopy is an important prerequisite for the diagnosis of cervical precancer. The gynecologist, alerted by abnormal cytology or a positive human papillomavirus (HPV) test or by a suspicious appearance to the cervix in the presence of negative cytology, is directed toward colposcopy, which facilitates an assessment of the distribution of precancerous epithelium within the cervix. As will be shown later, not only will the colposcope allow localization of the lesion but it will also aid in the selection of a biopsy site. It helps the clinician to select the treatment for cervical intraepithelial neoplasia, evaluate the very common subclinical and clinical papillomavirus infections, manage effectively the abnormal smear in pregnancy, and evaluate any extension of the precancerous lesion into the vagina. It does, however, require that the gynecologist has an understanding of the fundamental and varied physiologic processes that occur within the cervix at different times in a woman’s life. Thus, this chapter will not only detail the developmental anatomy and natural history of the different epithelial types but also examine them at times such as during adolescence, pregnancy, and menopause. The cervical epithelium is derived in fetal life from the Müllerian epithelium and the vaginal plate. The latter structure is attached to the urogenital sinus, which is believed to represent a modified Wolffian duct epithelium. There are two types of epithelium that exist in the fetal cervix: the columnar epithelium, derived from the Müllerian epithelium, and squamous epithelium, originating from the vaginal plate epithelium. These join at a fixed point at or just inside the cervical orifice; this point of union is called the original squamocolumnar junction. Present during fetal life, and thus deserving the term “original,” these two types of epithelium are referred to as the original columnar and original squamous. The columnar epithelium continues cephalad to the endometrium and extends caudally for a variable distance to border with the original squamous epithelium. The squamous epithelium is highly differentiated, stratified, and in turn extends upward from the junction with the vulval epithelium cephalad to its contact with the columnar epithelium. In some fetuses, the area between the original columnar and squamous epithelia is taken up by a third type of epithelium called the original metaplastic epithelium. This metaplastic epithelium seems to be of the same type and configuration as that found at other periods in the female’s life, such as during adolescence and pregnancy, i.e., times when the cervix is subjected to hormonal changes that induce epithelial modifications. It seems as though the hormonal changes present at such times result in columnar epithelium becoming exposed on the vaginal surface to an acidic environment; this event appears to be the stimulus for metaplastic transformation. The increase in estrogen secretion in late pregnancy, which also influences the cervix at puberty, seems to produce expansion of the cervical body. An eversion process takes place in which the columnar epithelium of the endocervix is exposed to the ectocervical acidic milieu. Examination using a vaginal speculum reveals a red area surrounding the external cervical os in later life; this corresponds to the area of everted endocervical tissue. Various terms have been used to describe this everted area: most commonly erosion, but alternate terms, such as erythroplakia, ectopy, and transformation zone, have also been used. The term transformation zone—that area enclosed by the original squamocolumnar junction—amply describes this region in which transformation between columnar and squamous epithelium occurs. The epithelium induced by the transforming process of metaplasia is called the metaplastic epithelium. It appears, as described above, in late fetal life, during adolescence, and in pregnancy. It is sometimes referred to as the typical, physiologic, or normal transformation zone. Within the transformation zone, a third type of epithelium, namely abnormal or atypical epithelium, develops; this is easily recognized colposcopically, and is related to the neoplastic process. It has distinctive colposcopic features, and within its tissues can be found not only physiologic epithelium but also the precursors and cursors of invasive squamous carcinoma of the cervix. The transformation zone containing this abnormal or atypical epithelium is called the atypical transformation zone. These epithelia are depicted in Figure 4.1. The changes of transformation alter the appearance of the cervix at various stages in the female’s life. In the prepubertal cervix there is minimal eversion compared with that occurring after puberty. In pregnancy, eversion with transformation dramatically alters the morphology of the different epithelial types. In contrast, during the menopausal era, there is inward retraction of the epithelium. All these periods will be discussed in more detail later. The original squamocolumnar junction, the permanent point of meeting between original squamous and columnar epithelia, outlines the lateral border of the transformation zone. It is fixed, but moves in relation to the whole cervix when eversion of the endocervical columnar epithelium occurs, as happens during adolescence and pregnancy. Figure 4.2a shows the likely positions of this junction. Position (1) corresponds to the view of the cervix as seen colposcopically in Figure 4.3. In this position the squamocolumnar junction is situated within the endocervix and the whole of the ectocervix is covered with original squamous epithelium. Position (2) in Figure 4.2a corresponds to the general view in Figure 4.4; the transformation zone is now obvious. The junction seems to exist halfway between the endocervical limit and the vaginal fornix. This appearance is also typical of the so-called ectropion or ectopy. The transformation zone in this situation is composed mainly of original columnar epithelium, although some small areas of metaplastic squamous epithelium have already developed. Figure 4.3 The cervix showing the squamocolumnar junction (1) in the endocervix, with original squamous epithelium (2) covering the ectocervix. Figure 4.4 The squamocolumnar junction (3) is at the mid-distance point between the endocervix and vaginal fornix. The original columnar epithelium is at (1), with a small island of metaplastic squamous epithelium at (2). Original squamous epithelium is at (4). Position (3) in Figure 4.2a corresponds to the general colposcopic view in Figure 4.5. In this situation, most of the ectocervix is covered by columnar epithelium and, as shown, may extend onto the vaginal fornix. The large transformation zone, with its basically columnar epithelium (Figure 4.5), will very quickly develop metaplastic epithelium, owing to its exposure to the vaginal acidic secretions that bathe the ectocervix. Figure 4.2b shows this situation; a physiologic transformation zone exists on the ectocervix and this is marked (A), the endocervix is at (1), and the original squamocolumnar junction is at (2). At (3) is the line of the squamocolumnar junction in a situation where columnar epithelium has extended onto the vaginal vault. This Müllerian duct epithelium extends into this situation in about 2% of normal females and is also found in those who have been exposed, during intrauterine existence, to non-steroidal estrogens. Such changes are also seen in cases of gynatresia. Figure 4.5 Colpophotograph showing an extensive transformation zone. The endocervical canal is at (1) with islands of metaplastic epithelium at (2) within areas of original columnar epithelium. The squamocolumnar junction is at (3) with a small longitudinal strip of immature squamous metaplastic epithelium (stippled) medial to this line, and extends posteriorly in the 6 o’clock position into the posterior vaginal fornix (toward 4). The original squamous epithelium is at (5). This condition is also called an ectropion or ectopy. The cervical epithelia have distinctive colposcopic appearances and each will be described separately. Colposcopically the original columnar epithelium exists in two general forms of arrangement. In the first relatively coarse subdivision it appears as two or three mounds or cushions which are called rugae on either lip of the cervix (around 2 in Figure 4.6a). Within the endocervix this arrangement is present in the form of longitudinal folds ((1) in Figure 4.6b), from which emanate smaller folds directed obliquely towards the internal os. Because of their general pattern, said to resemble the trunk and branches of a tree, they have been called arbor vitae. They have also been referred to as palmate folds or plicae palmatae. The regular arrangement of these folds can be seen clearly on a cross-section of the endocervix. Figure 4.6 (a) The “apparent” view of the transformation zone. (b) View of the endocervix. The light foreground is reflection artefact. In the second, much finer, grouping the folds are present as small pendulous areas resembling bunches of grapes ((2) in Figures 4.6, 4.7b). The basic subunit of the epithelium is the villus. The tissue is present either by itself or, more commonly, in combination with metaplastic squamous epithelium. Figure 4.6a shows the small individual grape-like villi present in the endocervical canal as well as on the ectocervix (in positions (1) and (2)). The squamocolumnar junction is at (3) and the original squamous epithelium is at (4). Figure 4.7(b) A single layer of “reserve cells” appearing conspicuously beneath the columnar mucinous endocervical cells (1). The origin of these cells is uncertain, but at this stage their detailed keratin signature already identifies them as parabasal or suprabasal squamous cells. They are present over the papillary surface and within the endocervical crypts or troughs. Their monotypic single-layer occurrence at one moment suggests a threshold crossed or a switch tripped over. Figure 4.7(c) A later stage of still incomplete squamous metaplasia with normal endocervical epithelium on the left at (1). Within the developing squamous epithelium there are residual columnar-lined surfaces (2) with endocervical cells. The original squamous epithelium ((4) in Figure 4.6a) is clearly identified as a smooth, usually featureless covering of the cervix; its uniform pink color contrasts with the redness of the original columnar epithelium. It joins the latter at the original squamocolumnar junction. The zone in which transformation will occur during fetal life, adolescence, and pregnancy is termed the typical or normal transformation zone. It is characterized by the presence of metaplastic epithelium, which may extend not only across the ectocervix but to within the endocervical canal. Transformation is more prominent at the squamocolumnar junction in an area where columnar epithelium is everted and exposed to the vaginal environment as seen in Figures 4.6a and 4.8. In Figure 4.6a the endocervical canal has been everted to show the transformation zone on the ectocervix. However, this is an artificial view, which has been called the “apparent view,” because, as the speculum is removed into the lower vagina, so the columnar epithelium of the transformation zone recedes into the endocervical canal (positions (1) and (2) in Figure 4.8). In the in vivo situation (Figure 4.8), no exposure to the vaginal environment has occurred and therefore no transformed epithelium (i.e., metaplastic epithelium) has developed. This is called the “real view.” The squamocolumnar junction is at (3) with the original squamous epithelium at (4). The columnar epithelium, being highly specialized and sophisticated, is preserved within the endocervical canal, away from the degrading influence of the vaginal environment ((1) and (2) in Figure 4.8). Figure 4.8 The “real” view of the transformation zone. Squamous metaplasia is preceded by the appearance of new cell types underneath the columnar epithelium, the so-called subcolumnar cells. These cells multiply and form a layer that eventually produces a normally differentiating squamous epithelium. The columnar epithelium sometimes remains atop these new squamous elements. They are eventually shed. These cells are believed to be pluripotent and are sometimes referred to as reserve cells. They are believed to replace continually the endocervical columnar epithelium that is lost in day-to-day wear. It has also been suggested that these cells may originate from the stroma. These would appear to be blood-borne monocytic-type cells that appear within the subcolumnar area and eventually develop in a similar manner to that described above for reserve cells. Morphologically, reserve cells have a somewhat similar appearance to the basal cells of the original squamous epithelium, in that they have round nuclei and little cytoplasm. During metaplastic development, the reserve cells multiply and form a layer that may be several cells thick; this gives rise to a distinct morphologic entity of reserve cell hyperplasia. The intermediate filament proteins, which are the important constituents of cytokeratin, are involved in providing internal stability within the cell as well as involvement in transport functions and gene regulation of the structure. The reserve cells are the progenitor cells of both immature squamous metaplasia and endocervical columnar cells. Keratin expression is identical in mature squamous metaplastic cells to that found in ectocervical epithelium. Associated with this stage is the thickening and fusing of the endocervical villi, a development that can be seen both histologically and colposcopically (Figure 4.9a–e). Figure 4.9 (a)–(e) The normal process of squamous metaplasia. The villi of columnar epithelium are replaced by metaplastic epithelium. The capillary structures of the stroma in the villi are compressed and reduced in height, ultimately forming a network under the epithelium; this is indistinguishable from the capillary network of normal squamous epithelium. Adapted from Kolstad P, Stafl A. Atlas of Colposcopy, 3rd edn. Edinburgh, UK: Churchill Livingstone, 1982, p. 58. In stage 1, there is a loss of translucency of the top of the villus, and the vascular structures within the villus become indistinct. This is shown in Figure 4.9a and corresponds to tissue seen at position (2) in Figure 4.7a. A histologic examination of the area will demonstrate a reduction in mucus content of the epithelial cells, which become flattened and cuboidal. This stage is followed by the appearance of multilayered undifferentiated epithelium, caused by the rapid division of the reserve cells. These eventually cap the villus and extend into the clefts between two adjacent villi, as shown in Figure 4.9b; colposcopically, this is represented by the tissue in position (3) in Figure 4.7a. Stage 2 is an extension of stage 1. More mature areas of squamous epithelium appear, in which individual opaque villi appear to be fused; the origin of the new surface is still apparent in this tissue (Figure 4.9c and the area between positions (3) and (4) in Figure 4.7a). Minute humps are also present and these represent the tips of the old villi. In stage 3, there is near obliteration of the original structure. This produces a smooth surface with multilayered and undifferentiated epithelium, into which has been incorporated the original vascular structures that existed in relation to the stromal core of the now fused villi. This is represented in Figure 4.9d,e, and in area (4) of Figure 4.7a. In this colpophotograph (Figure 4.7a), the original squamous (1) and columnar (2) epithelium with the original squamocolumnar junction (6) are seen. There are also many gland openings arrowed in the area of (5) and are associated with the intermediate forms of the process. Colposcopically, features of this process can be easily seen. First, the process starts at the tips of the villi, suggesting that the stimulus to this change is found in the external environment provided by the vagina. This influence seems to be the acidic pH of the vagina. Second, the essential process is an in situ transformation, so that the new epithelium emerges from beneath, and not by an ingrowth from, the original squamous epithelium. Third, the process proceeds from patches of already fused villi that are present within the unchanged columnar epithelium. The end result is a combination of epithelial patches, and this produces a superficial homogeneity, providing the covering to the transformation zone. As squamous metaplasia progresses, reserve cells undergo maturation, giving rise to recognizable immature squamous metaplasia (Figure 4.7b). While columnar cells remain on the surface of the newly developing squamous epithelium the metaplasia is said to be “incomplete.” This is seen in Figure 4.7c, in which there are residual glands and mucin-containing columnar cells (1) on the surface of the developing squamous epithelium (2). At this stage stratification is not apparent or may be just visible. This is shown in Figure 4.7d,e (high power), in which the original squamous epithelium is to the left again, incomplete squamous metaplasia is at (1), and stroma is at (2). The squamocolumnar junction is marked by a solid arrow. As the process continues further the epithelium loses its columnar surface and the mature squamous epithelium appears. This is shown in Figure 4.7f. The solid arrow separates the original squamous epithelium (1) with its usual pale glycogenated squamous layers from the metaplastic epithelium (2). There is striking epithelial scalloping or irregularity of the lower half of the epithelium (3) as a result of the intercalated stromal papillae, which were originally sited between the endocervical crypts and still contain capillary vascular channels. The new epithelium is very often darker with reduced glycogen levels and may remain so. The metaplastic process is stimulated by changes in the vaginal pH. It seems as though, especially in pregnancy, the vaginal pH causes destruction of the overlying columnar epithelium and generation of the reserve or subcolumnar cells. This can be seen in Figure 4.10a,b. In both these cervices, the metaplastic process is more intense the more peripheral the tissue is within the transformation zone. In Figure 4.10a, the original columnar epithelium at (1) contrasts with the immature squamous epithelium formed at (3). In Figure 4.10b, the intensity of the metaplastic process is apparent at the top of the villous process (3), again representing the portion of tissue most exposed to the vaginal pH. In Figure 4.10c the metaplastic process extends to the region of the internal os (1). Its upper extent is arrowed with the columnar epithelium appearing above this point and in the intervening so-called crypt spaces (2). This eversion process occurs predominantly in adolescence and during pregnancy, and shows the proclivity of the resultant exposed columnar area to undergo metaplasia. Figure 4.10 (a) The cervix of an adolescent (16 years) showing all the stages of squamous metaplasia. The endocervix with its original columnar epithelium is at (1). At position (2) is seen stage 2 of squamous metaplasia development with fusion of the villi, and at (3) this has extended to fusion of all the villi. Immature squamous epithelium is present, adjacent and medial to the squamocolumnar junction at (4). Between (2) and (3) it can be seen that the villous structure of the original columnar epithelium has been gradually lost until the surface has become flat with the development of more peripheral immature epithelium. This has developed in an area that is more exposed to the effect of the vaginal pH. The pallor of this immature epithelium (3) contrasts with the redness of the previous columnar epithelium (1). With further maturity, this pallor will be lost as the epithelium becomes thicker. (b) An area of the ectocervix showing the tendency for the metaplastic process to develop in areas exposed to the vaginal pH. Original columnar epithelium is seen at (2), emanating from the endocervix at (1) with early squamous metaplasia developing at (3) in the exposed areas atop the villous processes. The process of eversion with squamous metaplasia formation can easily be seen by close observation of the transformation zone in apparent and real views; these views imitate the normal eversion process. These processes are clearly displayed in Figures 4.11–4.13. In Figure 4.11, the cervix is seen in the apparent view, with the original columnar epithelium at (1), the original squamocolumnar junction marked with a dashed line, and the upper limit of the metaplastic process, the so-called new squamocolumnar junction, at (2). The original squamocolumnar junction is at (3), with original squamous epithelium at (4). Between positions (2) and (3), the squamous epithelium is pale and immature. As the speculum is withdrawn into the lower vagina (Figures 4.12, 4.13), imitating the in vivo position (real view) of the epithelial types, it can be seen that the original columnar epithelium recedes into the canal and all that is exposed on the ectocervix is immature squamous metaplastic epithelium (positions 2 and 3). In Figure 4.12, there is still some original columnar epithelium (1) exposed and the new squamocolumnar junction (2) remains obvious. However, upon full retraction (Figure 4.13) this junction is within the endocervix, leaving the newly developed immature squamous epithelium present on the ectocervix (between positions 2 and 3); this represents the area most in contact with the vaginal pH.
Colposcopy of the normal cervix: A prerequisite to establishing the diagnosis of cervical precancer
4.1 Introduction
4.2 Cervical epithelium: natural history
4.3 Cervical epithelium: topography
4.4 Cervical epithelium: colposcopic appearances
Original columnar epithelium
Original squamous epithelium
Transformation zone
Mechanisms involved in squamous metaplastic transformation
4.5 Squamous metaplastic epithelium
Colposcopic stages of development
Histologic features of development
Stimulus to development
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


