Clostridial Infections
Jose Zayas and Mobeen H. Rathore
CLOSTRIDIUM DIFFICILE
Clostridium difficile is a spore-forming, obligate anaerobic, Gram-positive bacillus, which can spread via the fecal-oral route. The most common manifestation of Clostridium difficile–associated disease (CDAD) is mediated by toxins A and B.1,2
 EPIDEMIOLOGY AND PATHOPHYSICOLOGY
EPIDEMIOLOGY AND PATHOPHYSICOLOGY
A recent U.S. study showed a rate of C difficile infection or colonization in hospitalized patients of 13 per 1,000, being one of the most widespread and serious healthcare-associated infections acquired in a hospital or long-term care facility.
Risk factors for developing CDAD include having gastrointestinal surgery, having prolonged stays in health care facilities, being immunocompromised, undergoing proton-pump inhibitor therapy, and having a history of cancer.1,2 Breastfeeding may offer some protective benefits. Treatment with antibiotics and other chemotherapeutic agents (eg, fluorouracil, methotrexate) can alter the natural GI flora and favor the emergence of C difficile.
CDAD results directly from toxin-mediated effects to the large intestine. The exact incubation period is unknown, but symptoms are known to develop up to 6 weeks after the discontinuation of antibiotics.1,2 Infants and children are more likely to carry C difficile asymptomatically in the GI tract than adults; it is estimated that 15% to 63% of neonates, 3% to 33% of infants and toddlers younger than two years of age, and up to 8.3% of children older than two years of age are asymptomatic carriers. Because the rates of symptomatic carriage (coexisting diarrhea) are not dissimilar to that for asymptomatic carriage, it is often difficult to establish a clear role for C difficile in causing mild GI disease in children. Over the past decade, more severe, sometimes fatal infection has been seen with outbreaks of C difficile infection caused by a virulent NAP-1/027 strain that appears to have an increased production of toxins A and B, fluoroquinolone resistance, and production of binary toxin. Infection is also more common in otherwise young, healthy individuals who have not been hospitalized or exposed to antibiotics.
 CLINICAL MANIFESTATIONS
CLINICAL MANIFESTATIONS
The presentation of CDAD can range from asymptomatic colonization to life-threatening disease. CDAD illness can include watery diarrhea, pseudomembranous colitis, toxic megacolon, perforation, and bacteremia with distant metastatic infections.3,4 Therefore, a high index of suspicion is critical, as is awareness of the limitations of current diagnostic tests.3,4
 DIAGNOSIS
DIAGNOSIS
The diagnosis of C difficile colitis should only be made if a toxin is found in the stool. Culture of the bacteria is not sufficient evidence to support the diagnosis. Commercially available enzyme immunoassays can detect both toxins A and B. Approximately 5% to 20% of patients, however, may require more than 1 stool assay to detect toxin.4 In the presence of suspicious symptomatology, toxin identified in a patient over 1 year of age is considered diagnostic for CDAD. When symptoms are severe, endoscopic evaluation can be diagnostic and is indicated even in the absence of positive toxin assays. There are typical findings or pseudomembranous colitis, characterized by the presence of an adherent inflammatory “pseudomembrane” overlying the mucosa (see Fig. 250-1).
 TREATMENT
TREATMENT
Antibiotics or chemotherapeutic agents should be immediately discontinued if possible. In approximately 20% of immunocompetent patients, CDAD will resolve within 2 to 3 days of discontinuing the offending agent. Additional antimicrobial therapy for CDAD is indicated for patients with immunodeficiency, severe disease or in whom diarrhea persists after antibiotics are discontinued. Strains of C difficile are susceptible to metronidazole and vancomycin, and both are effective for the treatment of mild CDAD.5 Metronidazole (30–50 mg/kg/day for 7 to 10 days) is the more cost-effective choice for the initial treatment of patients with CDAD. Vancomycin is indicated for patients who do not respond initially to metronidazole or in cases of severe CDAD.5,6 The recommended length of therapy is 10 to 14 days. Up to 40% of patients experience a relapse after discontinuing therapy, but the infection usually responds to a second course of the same treatment.5,6 Recent alternative treatment regimens include rifaximin, fiflazil, and nitaoxanide.7 Because the excretion of the organism and the toxin can be prolonged after clinical cure, follow-up testing for C difficile or its toxins is not indicated. Tapering or pulsing antibiotics on an alternative day basis during discontinuation of therapy, or administration of probiotics, may be useful to prevent initial occurrence and recurrence, but more data are needed to be certain. Antimotility agents should be avoided. Surgical intervention may be required in severe cases of CDAD unresponsive to medical therapy or to manage complications such as toxic megacolon or colonic perforation.
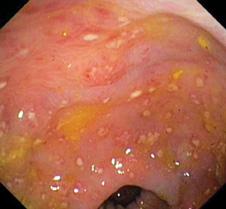
FIGURE 250-1. Colonoscopic image of pseudomembranous colitis in a patient with the acute onset of bloody diarrhea due to Clostridium difficile. Note the pseudomembranes adhered to the mucosal surface. (Courtesy of Colin Rudolph.)
 PREVENTION
PREVENTION
The first step in prevention is the judicious use of antibiotics. Contact precautions for patients with known or suspected CDAD should be implemented to prevent nosocomial transmission of C difficile. Importantly, commercial alcohol-based hand hygiene products (now commonly used in health care facilities as a substitute for soap and water hand washing) are not sporicidal and should not be relied upon to prevent person-to-person spread of C difficile infection.8
CLOSTRIDIUM BOTULINUM
Clostridium botulinum is a Gram-positive, obligate anaerobic bacilli most commonly found in the soil.9 Neuromuscular blockade by one of the neurotoxins results in a descending paralysis of varying degrees. Classically, three clinical presentations of botulism are described: infantile botulism, food-borne botulism, and wound botulism. Recently, inadvertent botulism has been described as an iatrogenic disease occurring in patients who have been treated with botulinum toxin injections for dystonia, other movement disorders, or for cosmetic procedures. C botulinum generates spores that survive extreme weather and temperature conditions. Unlike the toxin, which is heat labile, the spores are relatively heat resistant. Infantile botulism results from ingested spores of C botulinum that germinate in the colon and produce a neurotoxin. In food-borne botulism, it is the preformed toxin that is directly ingested from inappropriately handled food sources. The neurotoxin is absorbed and carried by the circulatory system to the peripheral cholinergic synapses. The toxin binds irreversibly and produces a flaccid paralysis by producing a presynaptic blockade, preventing the release of acetylcholine.9,10 The toxin also blocks acetylcholine from parts of the autonomic system inducing symptoms of dry mouth and reduced sweating.9
Infant Botulism
Infant botulism has been the most common form of botulism reported in the United States.10 There is no gender predilection. The average age of the onset of symptoms is 13 weeks of age. Infant botulism occurs at a significantly younger age in formula-fed infants compared to breastfed infants. There are no clear epidemiological risk factors for the development of infant botulism. Possible spore sources include foods, dust, and soil.9,10 Vegetables are the most common source for food-borne botulism in the United States.10 Although a history of honey ingestion is present in a minority of cases, children younger than 12 months should not be fed honey.11
 CLINICAL MANIFESTATIONS
CLINICAL MANIFESTATIONS
The classic presentation of an infant with botulism includes constipation, poor feeding due to a weak suck, a weak cry, and progressive descending weakness. Impaired respiratory effort may evolve to respiratory failure. In most cases, the source of spores is unidentifiable. Infant botulism displays a wide spectrum of presentations from transient mild weakness and hypotonia that may go unnoticed, to a fulminant, even fatal, illness. The first sign of the illness is constipation. Paralysis is usually symmetric and descending, beginning with the cranial nerves. This results in an expressionless face, ptosis, weak cry, and impaired gag or suck reflexes, which are followed by a generalized progressive hypotonia. Deep-tendon reflexes are normal initially but diminish later in the course of the illness. Despite a sad, lethargic appearance and a feeble cry, the infant conveys a paradoxical sense of alertness because the toxin does not cross the blood-brain barrier. The infant is usually afebrile unless the course is complicated by a secondary bacterial infection.
Infant botulism should be suspected in any infant who presents with a symmetric progressive weakness, poor feeding, and constipation. Clinical suspicion is the cornerstone of diagnosis, and the initiation of treatment should not be delayed awaiting laboratory confirmation. “Sepsis” is the most common admitting diagnosis. Other frequent admission diagnoses include dehydration, viral syndrome, and failure to thrive. The differential diagnosis includes hypothyroidism, metabolic disorders, drug or heavy-metal poisoning, myasthenia gravis, poliomyelitis, Guillain-Barré syndrome, Hirschprung disease, and Werdnig-Hoffmann disease.
 DIAGNOSIS
DIAGNOSIS
A stool specimen for toxin assay is the test of choice for diagnosis of infant botulism.
 TREATMENT
TREATMENT
In infant botulism, supportive care is the mainstay of therapy. However, the early use of human-derived botulism immune globulin (BABYBIG) is now standard. Specific treatment with BABYBIG is highly effective, shortening hospital stays from 5.5 to 2.5 weeks, and reducing morbidity and mortality. It is not recommended in other forms of botulism. Treatment with BABYBIG should be instituted as soon as possible and not delayed awaiting laboratory confirmation. BABYBIG immediately binds and neutralizes all circulating botulinum toxin and remains present in neutralizing amounts in the circulation for up to 6 months. This allows regeneration of nerve endings to proceed and leads to full recovery. Early treatment with BABYBIG within 0 to 3 days of admission shortens hospital stay by up to 1 week when compared to BABY-BIG administered at 4 to 7 days of admission.12
Non-Infant Botulism
 CLINICAL FEATURES
CLINICAL FEATURES
The symptoms of food-borne botulism begin several hours to days after the ingestion of a preformed toxin. Similar to infant botulism, these patients present with some degree of flaccid paralysis, a predominance of bulbar palsy, and absence of sensory nerve involvement. Early symptoms include blurred or double vision, dizziness, trouble swallowing, and difficulty speaking. A descending progressive paralysis may ensue. Because the toxin is in the gastrointestinal tract, decreased stool frequency and increased consistency is also a common feature. The neurologic manifestations of wound-associated botulism are indistinguishable from those seen in food-borne botulism; gastrointestinal symptoms are absent. Associated wounds are not necessarily outwardly impressive, although they are frequently deep and associated with avascular injuries. The average incubation period in cases of trauma is 7 days (range 4–21 days).
Inadvertent botulism demonstrates clinical characteristics consistent with naturally occurring botulism.10 Some patients develop associated autonomic nervous system effects following injections of toxin. Patients treated with toxin for cervical dystonia often experience dysphagia. This focal weakness likely results from the local spread of toxin from the injected muscles. Generalized weakness and autonomic symptoms are likely a result of circulating toxin in the blood.
 DIAGNOSIS
DIAGNOSIS
Routine laboratory tests are usually unremarkable. The tensilon test helps distinguish botulism from myasthenia gravis. In suspected cases, serum and stool samples should be sent for toxin confirmation. Detection of toxin in the patient’s serum, stool, wound, or food is diagnostic.
 TREATMENT
TREATMENT
Patients with suspected food-borne and wound botulism should be treated with bivalent antitoxin (types A and B) and possibly also monovalent type E antitoxin. Immediate administration of antitoxin is critical, because antitoxin arrests the progression of paralysis, although it does not reverse it.10 Theoretically, antibiotic usage may lead to lysis of C botulinum, releasing further neurotoxin, which could cause more prolonged or severe illness. Therefore, antibiotics should not be routinely employed. Aminoglycosides, due to their potential for additional neuromuscular blockade, should particularly be avoided because they may exaggerate the paresis. In the United States, food-borne botulism has been largely reduced by implementing safe canning and food manufacturing processes. To reduce wound-associated botulism, careful wound irrigation and basic wound care techniques should always be employed. The recent increase in inadvertent botulism cases underscores the importance of using botulism toxin only for clinically established and approved indications.
CLOSTRIDIUM PERFRINGENS
Clostridium perfringens is the most common cause of clostridial myonecrosis. It is also implicated in cellulitis, necrotizing fasciitis, food poisoning, and necrotizing enteritis (pigbel).
 CLINICAL MANIFESTATIONS
CLINICAL MANIFESTATIONS
Myonecrosis caused by Clostridial sp is called gas gangrene. C perfringens is readily found in soil samples, contaminated surgical and other objects, and the intestinal contents of animals and humans. It is also present in raw meat and poultry.13 Factors that may facilitate the growth of C perfringens include penetration of deep tissue, the presence of a foreign body, extensive tissue devitalization, tissue anoxia, polymicrobial infections, and an anaerobic environment. Necrotizing enteritis is a rare condition associated with β-enterotoxin produced by C perfringens type C. It occurs in children with protein-calorie malnutrition, or when diets contain trypsin inhibitors (sweet potatoes) or when infested by Ascaris parasites that secrete a trypsin inhibitor. Myonecrosis may occur after trauma, postoperatively, or spontaneously in the presence of another primary pathology. These toxins also have systemic effects such as direct cardiodepressive effects. Nontraumatic myonecrosis occurs occasionally from Clostridia in the gastrointestinal tract of immunocompromised hosts. Clostridial myonecrosis is heralded by acute and progressive pain at the site of injury with local, tense swelling, pallor, and a thin hemorrhagic exudate. Pallor gives way to a bronze or magenta discoloration, and hemorrhagic purplish bullae appear. Crepitus from gas production is suggestive but not pathognomonic and may not be present at all.14 A peculiar offensive odor, sometimes described as sweet, may be noted, with a brown serosanguinous discharge. Eventually, the muscle becomes “gangrenous”—black, friable, and liquefied. On the other hand, C perfringens can also cause a simple localized cellulitis indistinguishable from group A streptococcal cellulitis. Diagnosis is based on clinical manifestations, including the characteristic appearance of necrotic muscle noted at surgery. Systemic findings include tachycardia disproportionate to the degree of fever, pallor, diaphoresis, hypotension, renal failure, and changes in mental status. Untreated clostridial myonecrosis can lead to disseminated myonecrosis, suppurative visceral infection, sepsis, and death within hours.
Food poisoning caused by C perfringens is usually a self-limited illness due to the ingestion of the organism that elaborates enteric toxins. C. perfringens enterotoxin is responsible for the symptoms of common human food poisoning and acts by forming pores after interacting with intestinal tight junction proteins. Symptoms may also result from toxin ingestion itself. Typically, the illness starts 8 to 12 hours after ingestion of contaminated products containing high numbers of organisms. Symptoms usually last less than 24 hours and include nausea, severe abdominal pain, and profuse nonbloody, watery diarrhea. Fever is absent, and vomiting is uncommon. Necrotizing enteritis is a rare but life-threatening food-borne illness.15 Neonatal necrotizing enterocolitis may be caused by C perfringens, C butyricum, and C difficile. The role of these organisms needs further elucidation. Neutropenic enterocolitis (typhlitis) is a similar syndrome that occurs in the cecum of neutropenic patients. Clostridium septicum is the usual agent. Symptoms are fever, abdominal pain, and diarrhea. Initial treatment is with antibiotics, but surgical resection may be necessary.
 DIAGNOSIS
DIAGNOSIS
Although clostridial myonecrosis is a clinical diagnosis, early recognition is critical for a successful outcome. Myonecrosis and crepitance are classic findings but not pathognomic because infections with other organisms may also produce gas. Anaerobic cultures of wound exudate and blood should be performed. Tissue specimens and aspirates (not swab specimens) are appropriate for anaerobic culture. If gram-positive bacilli are present with a consistent clinical picture, the diagnosis of clostridial myonecrosis should be assumed until proven otherwise.
In food-borne illnesses, isolation of the same serotype from the stool is helpful in the diagnosis of food poisoning. However, for most cases of self-limited food poisoning, diagnostic testing is not indicated. Conversely, in cases of necrotizing enteritis, isolation of bacteria and toxin should be attempted from stool and any suspected food sources. Stool specimens, rather than rectal swab specimens, should be obtained.
 TREATMENT
TREATMENT
A high index of suspicion and immediate surgical excision of necrotic tissue and removal of any foreign material are essential to the management of clostridial myonecrosis. Decompressing fascial compartments is essential to prevent further tissue anoxia. Broad-spectrum antibiotics are indicated until culture and sensitivity reports allow for appropriate antibiotic adjustments given the prevalence of polymicrobial necrotizing infections.
High-dose penicillin G (250,000–400,000 U/kg per day, up to 24 million U/day, divided every 4 hours) administered intravenously has excellent activity against C perfringens and must be included in the initial antibiotic regimen.16 Approximately 5% of the clostridial species show variable degrees of resistance to penicillin. Clindamycin (40 mg/kg/day divided every 6–8 hours), can be considered as an alternative drug for penicillin-allergic patients or for treatment of polymicrobial infections. Polyvalent clostridial myonecrosis antitoxin has no proven benefit. Hyperbaric oxygen is favored by some experts as mode of therapy after surgical intervention. Clostridial cellulitis is treated by incision and drainage in addition to the antibiotics.
Treatment of food poisoning is symptomatic. Antibiotics are not indicated. However, necrotizing enteritis requires treatment with antibiotics (penicillin G, metronidazole). Up to 50% of seriously ill patients require surgery for perforation, persistent intestinal obstruction, or failure to respond to antibiotics.
For the prevention of myonecrosis, during initial wound management prompt and careful debridement, flushing of contaminated wounds, and removal of foreign material should always be performed. Clindamycin (20–30 mg/kg per day) may be of value for prophylaxis in patients with grossly contaminated wounds. In suspected hospitalized cases, isolation is not necessary.
For food-borne illness, prevention is achieved by cooking foods thoroughly and maintaining food at warmer than 60°C. Refrigerators should keep food cooler than 7°C (45°F).
REFERENCES
See references on DVD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


