Clinical Trials in Pediatric Oncology
Malcolm A. Smith and Gregory H. Reaman
Clinical trials have played a central role in converting many of the once fatal childhood cancers into conditions in which the vast majority of children with these diagnoses can be cured. For example, much of the progress in improving survival rates for children with acute lymphoblastic leukemia (ALL) can be attributed to the conduct of sequential phase III clinical trials in which more effective treatment approaches for children with ALL were reliably identified and carried forward as the standard of care that replaced less effective therapies. The end result of these series of clinical trials conducted over more than four decades has been the identification of treatments that produce much higher 5-year survival rates than those previously achieved (Fig. 446-1).
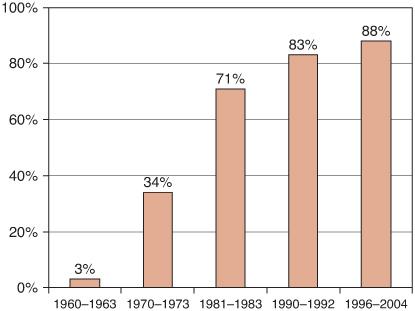
FIGURE 446-1. Five-year relative survival rates for children (< 15 years) with acute lymphoblastic leukemia.
Pediatric oncologists continue to feel that it is generally appropriate to offer families the opportunity to participate in clinical trials, because even the best currently available treatments are not optimal for many childhood cancers. For many cancers, a substantial proportion of children do not achieve long-term survival with current standard treatments. Furthermore, many treatment approaches cause short- and long-term treatment-related sequelae that reduce quality of life and survivorship. Although participation in clinical trials cannot provide guaranteed benefit to individual study subjects, the conduct of well-designed clinical trials addressing critical questions of therapy, most often linked to correlative biologic studies, remains the path forward for identifying more effective and less toxic treatments for children with cancer.
The general ethical principles that apply to research involving children are especially important in the pediatric oncology setting, given the vulnerability of children with these life-threatening diseases. Subpart D of the Code of Federal Regulations represents the regulatory embodiment of these principles, and institutional review boards are responsible for ensuring adherence to these principles. As cancer therapies evaluated in the research setting inherently involve greater than a minor increase over minimal risk, participation by children in cancer treatment clinical trials must offer the prospect of direct benefit as prescribed by 45 CFR 46.405 (http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.htm). The risks associated with clinical trial participation must be justified by the anticipated benefit, and the relation of benefit to risk must be at least as favorable as current treatment alternatives for the population being studied.
Phase I clinical trials for children with cancer are a key step in the introduction of new treatment approaches into the pediatric setting.1 The standard approach to these “first in children” clinical trials of novel anticancer agents requires previously defining the toxicity profile of the agent and establishing a recommended phase II dose for the agent in adult cancer patients. This adult experience allows the initial pediatric phase I study, conducted in children with resistant or refractory diseases, to begin at a dose near the adult recommended phase II dose and to include very limited dose escalation around this dose. Therefore, pediatric phase I trials usually involve far fewer dose levels than comparable trials in adults, and most pediatric phase I patients receive doses of the study agent that approximate the dose that will be used in subsequent phase II testing.2 When pediatric phase I trials are conducted with appropriate safeguards, treatment-related mortality is low. Although the primary objective of phase I trials is to define an appropriate dose and schedule for future testing, there is the potential for clinical benefit from participation in such trials.2
Phase II clinical trials are designed to identify activity signals for novel anticancer agents or combinations of agents in specific cancer diagnoses. The activity signal is generally defined in terms of objective response, which is measured either as shrinkage in tumor volume for children with solid tumors or as induction of remission for children with leukemia. The standard pediatric oncology phase II study design employs two stages of enrollment, with an initial stage of 10 to 15 patients and with continuation to a second stage of comparable size only if sufficient numbers of objective responses are identified in subjects enrolled in the first stage.3 For phase II studies evaluating the activity of a single novel anticancer agent, it is possible to attribute any objective responses to the agent under evaluation. However, when the goal of a phase II study is to identify the contribution of a novel agent when it is used in combination with other known active agents, it is necessary either to include a concurrently randomized comparator group treated only with the known active agents, or, alternatively, to compare the observed activity of the novel combination to that previously observed in an appropriate historical control population treated with the known active agents.4
Pediatric oncology phase III clinical trials compare the current best available therapy to a new treatment approach for which there is a body of evidence supporting potential superiority. These are complex studies, as standard treatment for most childhood cancers is multimodality, involving treatment with multiple chemotherapy agents as well as surgery and/or radiation therapy. Phase III trials generally enroll large numbers of subjects, with accrual targets ranging from several hundred to several thousand study participants. Most often, these studies target improvements in event-free survival, in which an event is defined as failure to achieve remission, disease recurrence, second malignant neoplasm, or death. The size of phase III studies is dependent on their targeted improvement in outcome and upon their false-positive error rates (a or type I error) and false-negative error rates (β or type II error), which are generally set at 5% and 10% to 20%, respectively. Because phase III clinical trials require a large number of study participants and because the number of children with any individual type of cancer is relatively small, pediatric oncology phase III studies require national (and sometimes international) participation. In North America, almost all phase III clinical trials are conducted through the Children’s Oncology Group, which at any given time has 20 to 30 actively enrolling phase III trials for the multiple types of cancer that occur in children. These clinical trials undergo multiple levels of scientific and regulatory review by childhood cancer experts as well as by the National Cancer Institute Pediatric Central IRB to ensure the importance of the research question and the validity of the methods being used to address the research question, and to ensure that study participants are appropriately protected from research risks. An important concept for phase III trials is that the research community is in equipoise regarding the treatment approaches being compared, with no compelling preference for one treatment or the other based on available data.5 During the conduct of phase III trials, data and safety monitoring committees are the bodies charged with confidentially examining accumulating outcome data and deciding whether study continuation is appropriated based on the protocol-specified monitoring guidelines.6
Risk-adapted therapy is a central concept in pediatric oncology phase III clinical trials. Progress over the past five decades has resulted in treatments that produce 5-year survival rates in excess of 90% for some diagnoses, including Wilms tumor, Hodgkin lymphoma, and neuroblastoma occurring in infants. However, some diagnoses continue to have very guarded prognoses, including metastatic sarcomas of bone and soft tissues, neuroblastoma occurring beyond infancy, and brain stem glioma or supratentorial high-grade glioma. Even children with the same diagnosis can have vastly different prognosis based on both clinical characteristics, as well as on biologic characteristics of their cancers. As examples, infants with disseminated neuroblastoma whose tumors have amplification of the MYCN gene have long-term survival of less than 30%, while similar infants with neuroblastoma whose tumors have normal MYCN gene number have long-term survival of greater than 90%. Likewise, children with acute lymphoblastic leukemia (ALL) and favorable biological features (eg, a chromosomal translocation involving the TEL and AML genes or extra copies of multiple whole chromosomes) have quite favorable outcome, while children with ALL and unfavorable biologic features (eg, a chromosomal translocation involving the BCR and ABL genes) have a much less favorable outcome. Clinical trials for children who have cancers with favorable prognosis are tailored to their more favorable outcome by evaluating modest modifications of standard therapy that seek to reduce treatment-related sequelae while maintaining favorable outcome. Conversely, clinical trials for children with cancers associated with an unfavorable prognosis often evaluate more aggressive treatment approaches or highly novel treatment strategies that seek to improve survival rates above the currently unacceptable low levels. All pediatric cancer clinical trials demand rigorous quality assurance of eligibility, adherence to protocol-specified treatment and evaluations, as well as validity of individual study subject data, which are incorporated in the final study database. Fastidious quality control guarantees the integrity of the science, which defines the standard of care.
Recent pediatric oncology clinical trials are increasingly designed to enroll children whose cancer cells have specific molecular characteristics, based on the premise that cancers with specific molecular abnormalities will be more likely to respond to molecularly targeted agents directed against these abnormalities. The prime example of this strategy is the assignment of children with ALL whose leukemia cells have the BCR-ABL fusion gene to clinical trials evaluating molecularly targeted agents (eg, imatinib or dasatinib) that inhibit the ABL kinase.7 As the number of molecular lesions identified in childhood cancers increases and as the number of molecularly targeted agents expands, this scenario will become more common. The potential of targeted therapy approaches to provide improved efficacy with reduction in toxicity risks is particularly relevant for infants, children, and adolescents with cancer.
REFERENCES
See references on DVD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


