Malformations of central pillar
Malformations of surrounding rings
Disturbance of axial component of occipital sclerotome, proatlas and C1 resegmented sclerotome
Disturbance of lateral component and hypochordal bows of proatlas and C1 resegmented sclerotome
Odontoid dysgeneses
Basioccipital dysgeneses
Proatlas anomalies
C1 sclerotome anomalies
Aplasia/hypoplasia of odontoid components
Failed midline integration of basioccipital primordium
Hyperplasia of hypochordal bow of proatlas
Aplasia of hypochordal bow of C1
Disturbance of odontoid synchondroses (IBZ)
Bifid clivus
Third occipital condyle
Aplasia and hypoplasia of anterior C1 arch
Os odontoideum
Basioccipital dysplasia
Pre-basioccipital arch
Aplasia/hypoplasia of lateral sclerotome of C1
Ossiculum terminale
Basilar impression
Hyperplasia of lateral (exoccipital) sclerotome of proatlas
Aplasia and hypoplasia of posterior C1 arch
Abnormal resegmentation of proatlas centrum
Platybasia
Hypertrophic occipital condyle
Aplasia of lateral mass of C1
Os avis
Retroflexed dens
Non-resegmentation of proatlas (anterior homeotic transformation)
Combined C1 hypochordal bow and lateral sclerotome dysplasia
Failed midline integration of basal dental segment
Basilar invagination
Atlas assimilation
Aplasia of lateral mass and anterior C1 arch
Bifid dens
Basilar kyphosis
Posterior homeotic transformation
Combined anterior and posterior C1 arch defects
Short clivus
Bifid anterior and posterior C1 arch
This chapter deals first with the normal development and genetic control of the bony CVJ, followed by descriptions of congenital malformations of this region with emphasis on their embryogenesis and clinical relevance. Special considerations of the techniques and pitfalls of fusion and decompression of the occiput-C1–C2 complex in young children are provided when applicable.
Embryology of the Craniovertebral Junction
The Pre-somitic Stage
At gastrulation, epiblastic cells from the embryonic plate caudal to the head process invaginate through the primitive streak to form mesoderm on each side of the neural plate, while cells from both sides of the dorsal lip of Hensen’s node migrate through the primitive pit to integrate into the midline notochord. The embryonic plate thus elongates by new additions to its rear (caudal) aspect [24].
The anterior-posterior (rostrocaudal) polarity of the embryo is determined very early during gastrulation. The prechordal mesoderm rostral to the otic vesicle (and notochord) forms most of the bones and muscles of the head and face without ever developing somites. Caudal to this tissue is the somitic region which extends along the body axis down to the tip of the tail (Fig. 1). The anterior somitic region from the otic vesicle to the blastopore (future anus) corresponds to the future body axis from the occiput to the anus. Here, the epiblastic cells condense to form the parachordal mesoderm on each side of the notochord following systematic ingression movements through the primitive streak. This initially homogenous column of cells, also called pre-somitic mesoderm (PSM) or segmental plate, subsequently segregates into segmental clusters called somites, which will eventually give rise to the smooth muscle of the dermis, the axial musculature, the vertebral column, and support structures of the peripheral nervous system. After blastopore closure and complete regression of the primitive streak, the gastrulating region for the tail is restricted to a small region called the tail bud, located at the caudal tip of the primitive streak. The tail bud functions as a blastema of undifferentiated cells [20, 21]. The pre-somitic mesoderm and later somites of the caudal and tail regions are therefore not formed by epiblastic ingression but by progenitor condensation in situ.
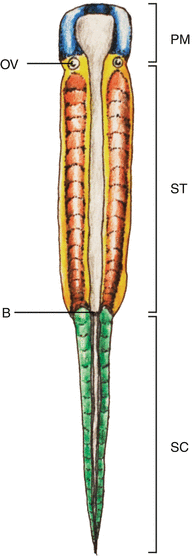

Fig. 1
Vertebrate embryonic plate around gastrulation showing the three main regions of the body plan: The prechordal mesoderm (PM) is anterior to the otic vesicle (OV). Posterior to the OV to the blastopore (B) is the somitic region of the trunk (ST), caudal to which is the caudal (tail) somitic region (SC)
Primary Segmentation: Somitogenesis
Prior to the appearance of somites, the PSM remains a loose mesenchyme without specific cellular polarity or stratification pattern alongside the lengthening notochord and neural plate [75]. During somitogenesis, the loose mesenchymal cells of the PSM undergo transformation into tightly-apposed epithelial cells with definite polarity and orientation. A newly formed somite is a compact epithelial sphere (“somitomere”) composed of a single layer of radially arranged cells with apices pointing towards a central lumen (somitocoele), which contains a few mesenchymal cells [45] (Fig. 2). The epithelial cells have polarised Golgi zones, basally aligned nuclei, and actin molecules near the luminal border, the entire sphere being enveloped by a collagen-containing basal lamina [4]. This epithelial conversion of the PSM is aided by an increase in cell-cell adhesion mediated by a sharp but transient rise in the levels of the calcium-dependent adhesion molecule N-cadherin, of fibronectin and possibly cytotactin [88].
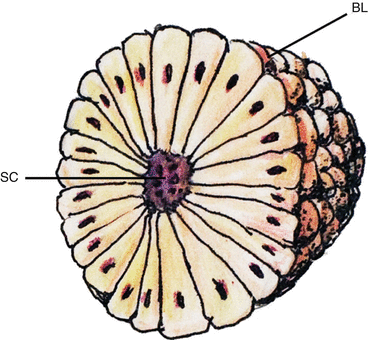

Fig. 2
Somite with radially arranged and polarised epithelial cells surrounding a central cavity, the somitocoele (SC) and enveloped by a basal lamina (BL)
Somitogenesis begins soon after internalisation of the prochordal (head) mesoderm and continues through subsequent production of the body axis. The first somite forms immediately caudal to the otic vesicle [38], followed by sequential transformation such that a new pair of somites is regularly added in a rostrocaudal direction until a fixed species-specific number of somites is reached (Fig. 3). Thus, depending on the stage of gastrulation, the growing column of body mesoderm consists of a rostral section of mature somites already undergoing differentiation into sclerotomes and dermomyotomes, a middle section of new pre-differentiated epithelial somites, and a caudal section of pre-somitic mesoderm just rostral to the remaining primitive streak, the whole enterprise necessarily evolving in parallel with the neurulating neural plates (Fig. 4).
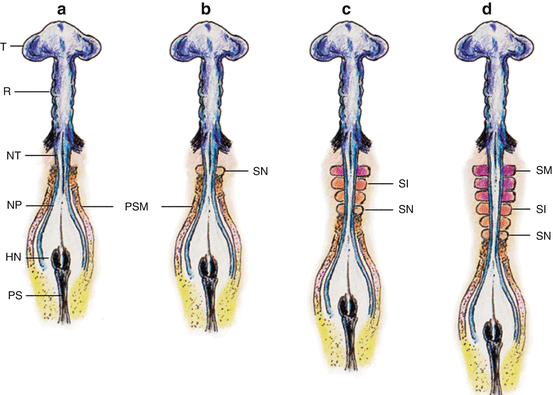
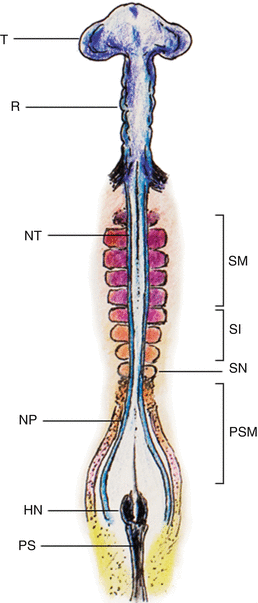

Fig. 3
Primary somitogenesis in a chick embryo. (a) At very early gastrulation, the mostly unneurulated neural plate caudal to the prochordal plate (head process) is flanked on each side by the pre-somitic (pre-segmented) mesoderm or PSM. (b) The first pair of somites is formed just caudal to the otic vesicles. The PSM column elongates from addition of new cells from the caudal embryonic pole rostral to Hensen’s node. (c) The new somites (SN) are formed in a rostrocaudal direction so that the older more matured somites (SM) are rostral, i.e., closer to the cephalic end of the embryo. (d) The older matured somites (SM) have undergone dorsoventral differentiation (purple colour) into sclerotome and dermomyotome. The intermediate-aged somites (SI) (orange colour) are pre-differentiated units without dorsoventral specification. The PSM is always at the most caudal end closest to Hensen’s node. Rostrocaudal sequential somitogenesis parallels progression of primary neurulation. HN Hensen’s node, PS primitive streak, T telencephalon, R rhombencephalon, NT neural tube, NP neural plate

Fig. 4
Chick embryo during sequential somite formation close to the end of gastrulation. The rostral-most mesoderm consists of matured somites (SM) already having undergone dorsoventral differentiation into dermomyotome and sclerotome, flanking the formed neural tubes (NT); followed by the newer intermediate-aged pre-differentiated epithelial somites (SI), the new somites (SN), and the pre-somitic mesoderm (PSM) on each side of the unneurulated neural plate (NP). HN Hensen’s node, PS primitive streak, T telencephalon, R rhombencephalon
Current thinking regarding the mechanism of metameric transformation of the PSM follows what is called the “Clock and Wavefront” model, first proposed by Cooke and Zeeman in 1976. In this model, PSM cells oscillate between a permissive and a nonpermissive state for somite formation. These oscillations are phase-linked and controlled cell-autonomously by a “Segmentation Clock”. Somitic formation is triggered when cells of the rostral PSM, while in the permissive phase of the Clock, are hit by a wavefront of maturation (or determination) that slowly moves caudally along the embryonic axis (Fig. 5). At this exact site, a transverse column of “boundary cells” forms across the width of the PSM, such that at the next periodic activation of the “Segmentation Clock”, a consecutive column of boundary cells, together with the previous boundary, now segregates out a segment of PSM that will ultimately evolve into a somite. Thus, the Clock generates a temporal periodicity that is translated spatially into the periodic boundaries of the somites [75] (Fig. 6).
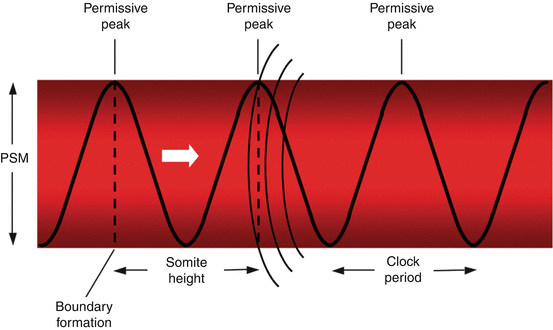
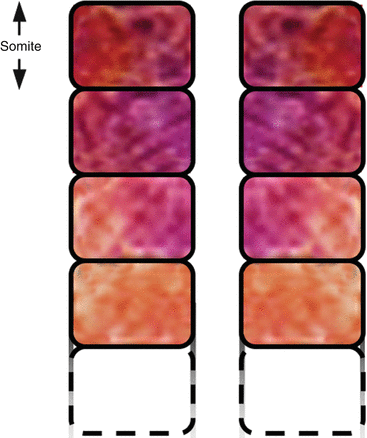

Fig. 5
The clock and wavefront theory of primary somitogenesis. The Segmentation Clock is a pattern of cell-autonomous, phase-linked oscillations of certain “cycling” gene expression within the PSM cells, producing a sinusoidal wave of peaks and troughs of gene products which render the cell either “permissive” or “nonpermissive” to changes when “hit” by a moving wavefront. Block arrow shows direction of wavefront. When the wavefront (curved lines) hits the “permissive” peak of the gene cycle, the cells at that region of the PSM undergo changes which demarcate them from the surrounding uncommitted mesenchyme, causing formation of a boundary zone (dotted vertical lines) cutting across the whole width of the PSM column because the clock periods (time lapses between wave peaks) in the PSM are in synchrony. The distance between two boundary lines thus represents the height of a somite, which, in turn, depends on the clock period and, to a certain extent, the velocity of the moving wavefront. Thus, in the clock and wavefront model of primary somitogenesis, time, in terms of the clock period, is transformed into space, in terms of the somite size. The Clock periodicity is species specific

Fig. 6
Periodic boundary formation across the width of the PSM column along its rostrocaudal axis causes segregated somites to form sequentially. The distance between two adjacent boundaries represents the longitudinal dimension of a somite
At the molecular level, the phasic oscillation of the Segmentation Clock is reflected by rhythmic expressions of a class of genes called cycling genes [74]. These genes include the c–hairy1 family in fish, chick, frog, and mouse, which encode transcription factors such as the split (HES) family and the glycosyltransferase Lunatic Fringe [28, 56], all tightly involved in the Notch signalling pathway and its ligands, suggesting that periodic Notch activation plays a critical role in the oscillator (Fig. 7). Also, these genes oscillate largely in synchrony in the PSM, suggesting they are downstream of a common cycling activator.
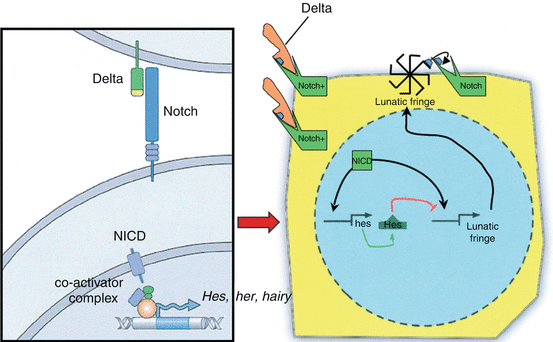

Fig. 7
The molecular basis of the Segmentation Clock. A ligand (e.g., Delta) is bound to the surface Notch signalling receptor, whose intracellular domain (NICD) then detaches from the cell membrane and enters the nucleus, where it coactivates cycling genes of the C–hairy 1 family, encoding transcription factors such as HES, HER, HAIRY, and Lunatic Fringe. In chick, Lunatic Fringe is known to recycle back to the cell surface, where it “enables” the surface Notch signalling receptor to accept another ligand. The repetitive working of this simplified cycling gene model is the underlying mechanism of the oscillating Segmentation Clock
In chick and mouse embryos, the maturation or determination wave is generated by the expression of genes which include the fibroblast growth factor gene fgf8 [82]. It is known that the caudal domain of the PSM is very high in the factor FGF8, which seems to actively maintain the mesenchymal identity of caudal PSM cells. The FGF8 gradient decreases towards the rostral axis, so that at the rostral domain of the PSM where somitogenesis is taking place, FGF8 level is very low. Also, overexpression of fgf8 in this region results in inhibition of somitogenesis. Thus, the activation of epithelisation of PSM cells is negatively regulated by FGF8, whose absence allows the PSM cells to become competent to respond to the clock signal and initiate somitic boundary formation [26]. The limit between the two domains of high (in the caudal PSM) and low (in the rostral PSM) fgf8 expression therefore marks the determination wavefront of somitogenesis [75] (Fig. 8).
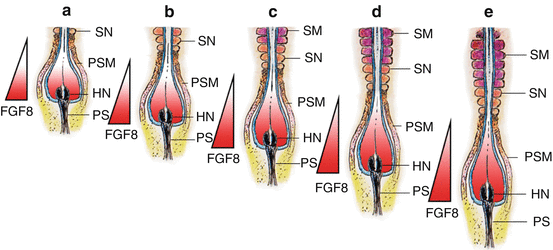

Fig. 8
The molecular basis of the somitogenesis wavefront. Boundary formation at the PSM is linked to expressions of fibroblast growth factor genes, especially fgf8. High concentrations of FGF8 maintains PSM cells in a primitive mesenchymal state, but a low concentration of FGF8 permits PSM cells to form boundary during the Segmentation Clock peak. Thus, the epithelial transformation (into somites) of PSM cells is negatively regulated by fgf8. High concentration of FGF8 is expressed by newly formed PSM cells at Hensen’s node, and low concentration is expressed in “older” PSM cells near the somitogenesis front. Thus, the limit between the high- and low-fgf8 domains near the site of active somitogenesis represents the somitogenesis wavefront (Represented by the apex of the FGF8 triangle). Figure a–e represent progressive stages of somite formation, elongation of the embryo, and caudal movement of the FGF8 wavefront. Because new PSM cells are continually being formed near Hensen nodes and the PSM column constantly elongates and gets ever “older” near the last formed somite, the wavefront also moves caudally. High concentration of FGF8 is indicated by deep red colour and low concentration by pale grey to white. The moving FGF8 gradient is also displayed by the coloured triangle. HN Hensen’s node, PS primitive streak, PSM pre-somitic mesoderm, SN new somite, SM mature somite. Significance of somite colours as in Fig. 3
Due to the constant caudal elongation of the body axis during gastrulation and the addition of new PSM cells with strong fgf8 expression to the rear, the FGF8 gradient is continuously displaced caudally. Accordingly, the determination wavefront also moves slowly in a caudal direction along the body axis. This ensures that the old and new metameric boundaries, at both ends of a new somite, are separated by a distance corresponding to the caudal displacement of the determination wavefront during one period of oscillation of the Segmentation Clock [74] (Fig. 8). The speed of new somite production is thus linked to the Clock period, which is species specific: 30 min for zebra fish, 90 min for chick, and 120 min for mouse [70].
Differentiation of the Somitic Mesenchyme
Within hours after its formation, each somite starts differentiating along its dorsoventral axis. Cells from its ventromedial part lose their epithelial arrangement and migrate towards the notochord to form, with the luminal cells, the mesenchymal sclerotomes [12, 45]. Together with the notochord, the sclerotomes provide exclusive material for the vertebral column. Cells from the dorsolateral part of the somite retain their epithelial arrangement to produce the dermomyotomes (Fig. 9a, b). The dermomyotomes later subdivides into the dorsal dermatome immediately beneath the ectoderm to give rise to the dermis and its smooth muscles, while the disaggregated cells between the dermatome and the sclerotomes remain closely packed as the myotome, forming ultimately the axial skeletal muscles [10, 12, 24] (Fig. 9c, d).
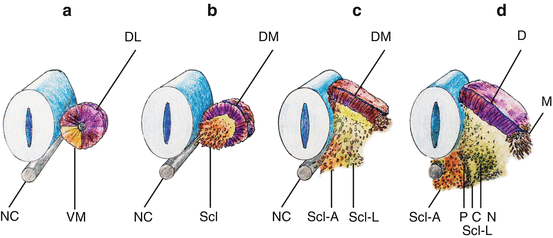

Fig. 9
Dorsoventral differentiation of somite. (a) Epithelial somite shows ventromedial cells (VM) destined to form the sclerotome. (b) Ventromedial sclerotome cells (Scl) de-epithelise from the somite and migrate towards the ventral notochord (NC). (c) Sclerotomal cells further subdivide into an axial cluster (Scl–A) surrounding the notochord and lateral paired clusters (Scl–L) flanking the perichordal axial sclerotome. Dorsolateral somite retains its epithelial pattern to become the dermomyotome (DM). (d) The lateral sclerotome (Scl-L) forms a triangle next to the axial sclerotome. The three sides of the triangle become anlagen for the pedicle (P), neural arch (N), and the costal process (C), respectively. The dermomyotome also subdivides into the dermatome (D) and the lateral migrating myotome (M)
While specification of the anterior-posterior pattern of the somite appears to be determined very early [1, 39, 46], the dorsoventral values are not intrinsic to the somites. Thus, if the somite were to be surgically rotated dorsoventrally by 180º, sclerotomes still develop in the ventromedial position while the dermomyotomes remain dorsolateral. Moreover, a dorsally implanted notochord represses dermatomal differentiation but encourages sclerotomal formation dorsally, while notochord ablation completely prevents sclerotomal appearance [24] (Fig. 10). These findings suggest that the dorsoventral differentiations of the somite depend on appositional instructions from the notochord, very possibly through sonic hedgehog (Shh) expression [48] (Fig. 11).
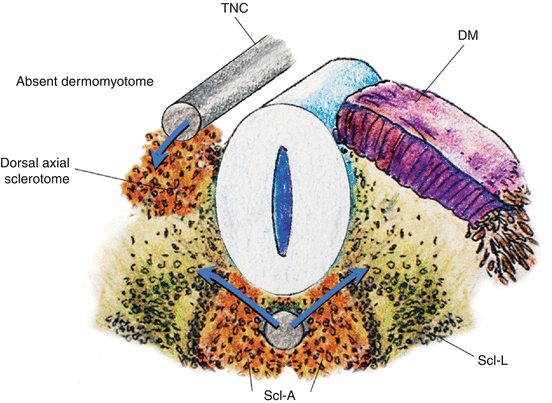
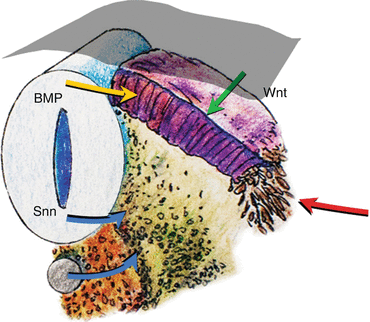

Fig. 10
A transplanted notochord (TNC) to the dorsal aspect of the somite induces sclerotomal differentiation dorsally and represses dermomyotomal formation on the side of the transplant, indicating inducers from the notochord direct dorsoventral specification of the somite. DM dermomyotome, Scl–A axial sclerotome, Scl–L lateral sclerotome

Fig. 11
Expressions of sonic hedgehog gene (shh) (blue arrows) from the notochord and floor plate of the neural tube signal sclerotomal differentiation and ventral migration from the somite. Bone morphogenetic protein (BMP) (yellow arrow) expressed by the roof plate and Wnt signalling (green arrow) from the ectoderm (grey mantle) induce dermomyotome differentiation. Other lateralising signals (red arrow) further encourage myotomal cell development
“Resegmentation” of the Sclerotome
The term “resegmentation” was originated by Remak in 1855 (“Neugliederung”) [81] and remains still a subject of controversies [16, 24]. It refers to the fact that the early metameric boundaries between the somites are once again changed and “reshuffled” during the development of the sclerotome, so that the later boundaries between the vertebral bodies do not match up with the original intersomitic clefts [12].
During somitic differentiation, the ventromedial cells destined to become sclerotome subdivide into paired lateral clusters flanking the ventral aspects of the neural tube, and an unpaired median (axial) cluster surrounding the midline notochord (Fig. 12). The formation of the vertebral column takes place first in the lateral sclerotome, where a conspicuous subdivision into a densely packed caudal half and a more loosely cellular cranial half soon appear, separated by a fissure (of von Ebner [27]). The loose cranial half attracts, promotes, and supports the growth and expansion of peripheral nervous tissue from the neural tube and neural crests, but itself never chondrifies into vertebral parts (Fig. 13, middle). In contrast, the caudal densely-packed mesenchyme of the lateral scleroderm soon takes on a triangular shape. The side of the triangle facing the axial perichordal sclerotome, which will become the future vertebral body, gives rise to the pedicle. The side facing dorsolaterally away from the axial sclerotome forms rudiments of the neural arch, and the side of the triangle facing ventrolaterally becomes the costal process (Fig. 9d).
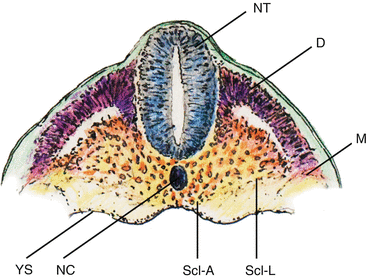
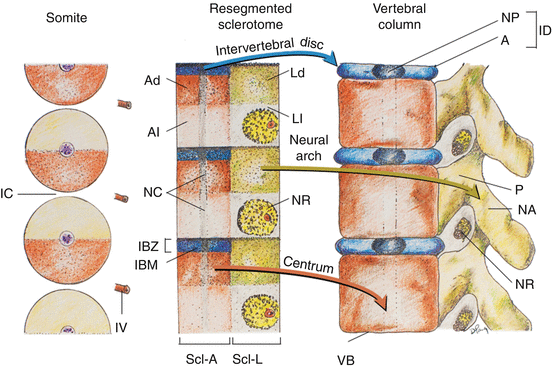

Fig. 12
Coronal section of chick embryo after dorsoventral differentiation of somites, showing axial or perichordal sclerotome (Scl–A) surrounding the notochord (NC) and lateral sclerotome (Scl–L) on both sides of the neural tube (NT). D dermatome, M myotome, YS yolk sac membrane

Fig. 13
Resegmentation of somites to form sclerotomes and changes of sclerotomal primordia to mature vertebral parts. The somitic and primordial origins of vertebral parts and phenotypic parts are colour-matched, and the locations of the somites, resegmented sclerotomes, and vertebrae along the embryonic axis are approximately counter-registered. During resegmentation, the sclerotome is formed from the caudal and rostral halves of two adjacent somites, such that the middle of the resegmented sclerotome lines up with the intersomitic cleft (IC). Both the axial sclerotome (Scl–A) and lateral sclerotome (Scl–L) develop dense and loose zones. The dense zone of the lateral sclerotome (Ld) becomes the neural arch (NA) and pedicle (P), which is attached to the rostral part of the vertebral body (VB) formed from chondrification of the loose (Al) and part of the dense zones (Ad) of the axial sclerotome. The rostral layer of the dense zone of the axial sclerotome soon forms the intervertebral boundary zone (IBZ) containing intervertebral boundary mesenchyme (IBM), which ultimately forms the annulus (A) and, together with notochord remnants (NC), the nucleus pulposus (NP) of the intervertebral disc (ID). The loose zone of the lateral sclerotome (Ll) does not form bone but promotes emergence of the nerve roots (NR). Thus, the neural arch is derived from a single somite, but the vertebral body receives contributions from two adjacent somites. IV intersomitic vessel. Arrows indicate developmental fates of the sclerotomes
Shortly after the specifications of the lateral sclerotome into its arcual, costal, and pedicular components, the initially loosely-meshed mesenchyme within the perichordal axial sclerotome also begins to show compartmentalisation. A cell-dense zone develops at the same level as the dense caudal half of each lateral sclerotome, partly due to medial expansion of the lateral band of condensed tissues from the lateral sclerotome. The axial sclerotome in between these median dense zones remains loosely cellular. Later, the cranial-most layer of the axial dense zone, in line with von Ebner’s fissure in the lateral sclerotome, becomes even more tightly packed and forms the intervertebral boundary zone (IBZ) (Fig. 13, middle). This intervertebral boundary mesenchyme (IBM) ultimately forms the ringlike annulus fibrosus of the intervertebral disc, permanently enclosing a looser central core of nucleus pulposus made partly of notochordal remnants. The vertebral body itself is mainly made from chondrogenesis in the loose-cell zone of the perichordal sclerotome, now called the prevertebra [11, 12, 24, 67], although contributions from the condensed tissue adjacent to the IBZ have been observed [16, 23]. As a final step, the pedicular anlagen from the lateral sclerotomes fuse with the chondrifying prevertebra of the axial sclerotome, while the neural arches surround the neural tube to complete the vertebral ring (Fig. 13, right). The costal component becomes the future transverse process but only in the thoracic region is it predestined to form ribs.
Thus, the neural arch of the vertebra is derived from the caudal-lateral part of a single somite [16]. However, labelling and transplantation experiments of half-somites have repeatedly demonstrated that each vertebral body is made up of cells from the axial zones of two adjacent somites [2, 3]. Although the exact boundary of individual somite participation is not known [12], the juxtapositioning of axial and lateral sclerotomal components suggests that each vertebra probably comes from the caudal half of one somite and the rostral half of the somite below [67]. This will explain the slightly off-step registration between the levels of the somite and the resegmented sclerotome [12, 17, 67] on the embryonic axis such that the middle of the resegmented sclerotome lines up with the original intersomitic cleft (Fig. 13, left and middle). Also, since the dense portion of the lateral sclerotome is in line with the dense zone of the axial sclerotome adjacent to the IBZ, it makes perfect sense that the mature pedicle is joined to the cranial and not the caudal half of the vertebral body [12]. It also follows that the spinal nerve, ganglion, and blood vessel from the corresponding somitic segment, being associated with the loose cranial half of the lateral sclerotome, must cross above its own neural arch and that the corresponding segment of the spinal cord is always slightly more rostral to its companion vertebral body (Fig. 13, right). Given there are eight cervical somites but only seven resegmented axial sclerotomes and consequently seven cervical vertebrae, the C1 nerve root emerges above the C1 neural arch, and the C8 nerve root comes through below the C7 neural arch and above the T1 neural arch, which in fact is derived from the C8 somite. Finally, resegmentation of the sclerotome explains why the original “pre-resegmented” intersomitic vessel ultimately enters the midpoint of the vertebral body as the segmental nutrient artery.
Special Developmental Features of the CVJ
The CVJ is the product of the occipital somites and the first three cervical somites. There is controversy regarding the proper number of occipital somites in vertebrates. Wilting et al. and others [14, 80, 99] thought there were five occipital somites in the chick and mouse but conceded that the first somite either disappeared early or was an insignificant clan of cells that lacked sclerotomal lineage. Müller and O’Rahilly [67] studied staged human embryos and concluded that, in humans, there are only four occipital somites that participate in formation of the skull base. The transitional zone between the skull and the cervical spine is thus taken to be between the 4th and 5th somites. During the fourth week of gestation, there are consequently 4 occipital, 8 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 8–10 coccygeal somites, 42 pairs all told.
Occipital Somites (Somites 1–4) and the Proatlas
Following the general scheme, the first three occipital somites give rise to an axial perichordal sclerotome and a lateral sclerotome, but no resegmentation takes place here. The axial sclerotomes never subdivide into dense and loose zones and therefore no intervertebral boundary mesenchyme exists. They all eventually fuse into a unit which later chondrifies to become the rostral basioccipital [67]. The first three lateral occipital sclerotomes, like the vertebral sclerotomes, form dense and loose zones, and the loose zones of the second and third lateral occipital sclerotomes foster expansion of the upper and lower hypoglossal nerve roots and artery, while the corresponding dense zones form the bony hypoglossal canal.
Unlike the first three occipital somites, the fourth occipital (O4) somite does show resegmentation. Its caudal dense zone combines with the cranial loose half of the first cervical somite to produce the transitional sclerotome called the proatlas [17, 53, 61, 62, 66, 67] (Fig. 14, left and middle). The cranial region of the axial sclerotome of the proatlas soon fuses with the other three axial occipital sclerotomes to become the basion of the basioccipital [66], but its most caudal portion, probably derived from the 1st cervical somite (somite 5), forms the anlage for the apical segment of the dens. Late in resegmentation, a boundary zone appears between this apical dental centrum and the loosely cellular prevertebra of the basioccipital, and the former soon detaches from the basioccipital and eventually becomes joined to the basal segment of the dens to complete the dental pivot (see below) [67, 99] (Fig. 14). Herein lies the most unique feature of the transitional zone of the CVJ between somites 4 and 5: unlike other IBZs that form intervertebral discs, downstream activity of the proatlas’ IBZ includes a physical severance of cells from the immediately adjacent loose perichordal zone of the basioccipital. The severance line appears to go through the original resegmentation fronts of the adjacent somites 4 and 5, so that the final cellular separation occurs right through the junction between the basion and the apical segment of the dens, both derived from the axial portion of the proatlas, which in turn comes from a combination of the caudal half of somite 4 and the rostral half of somite 5 (Fig. 15). This action, no doubt mediated by special cleavage genes (see below), not only allows the skull to become independent from the vertebral column, but also the separation and descent of the primordium for the apical dens from the basiocciput, to complete the final installation of the dens-axis assembly.
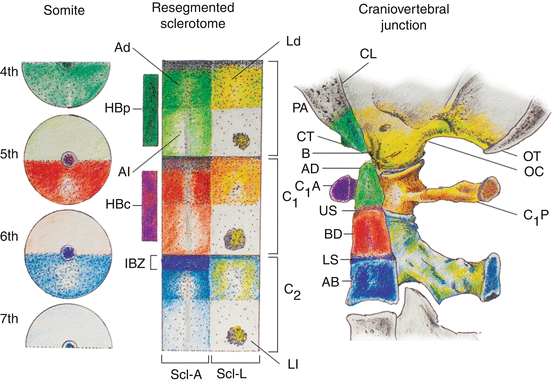
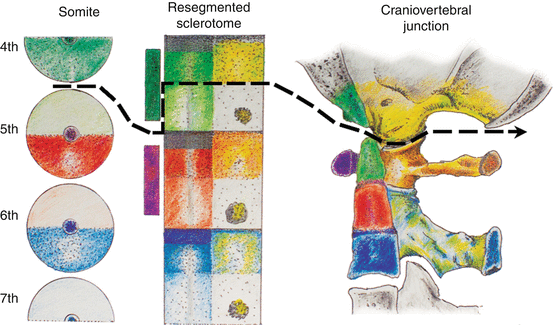

Fig. 14
Formation of the human craniovertebral junction. Sclerotomal primordia and their vertebral phenotypes are colour-matched. During resegmentation, the caudal half of the 4th somite (4th occipital somite) and rostral half of the 5th somite combine to form the proatlas sclerotome (PA). Derived from the proatlas are the axial zones (Ad and Al) which become the basion (B) of the basioccipital or clivus (CL) and the apical segment of the dens (AD), the lateral dense zone (Ld) becomes the exoccipital comprising the occipital condyle (OC), and lateral rim and opisthion (OT) of the foramen magnum; the proatlas’ hypochordal bow (HB p ) forms the ventral clival tubercle (CT). The C1 resegmented sclerotome (C 1 ) comes from adjacent halves of the 5th and 6th somites. Derived from the C1 sclerotome are: the axial zones form the basal segment of the dens (BD); the lateral zone forms the posterior atlantal arch (C 1 P); and the hypochordal bow (HB c ) forms the anterior atlantal arch (C 1 A). The C2 resegmented sclerotome (C 2 ) comes from the 6th and 7th somites. From the C2 sclerotome: the axial zone forms the C2 vertebral body (AB); the lateral zone forms the neural arch of C2 vertebra. The intervertebral boundary zone (IBZ) between the proatlas and C1 sclerotome forms the upper dental synchondrosis (US), and the IBZ between the C1 and C2 sclerotomes forms the lower dental synchondrosis (LS)

Fig. 15
The severance line, which results in final cellular separation of the skull from the cervical spine, runs through the original resegmentation fronts of the adjacent somites 4 and 5, corresponding to the junction between the basion and apical segment of the dens, and between the exoccipital, or future occipital condyle, and the lateral mass of C1, which is derived from the lateral portion of the C1 resegmented sclerotome
The lateral dense region of the proatlas becomes the two exoccipitals, which later form the two occipital condyles and the remainder of the anterolateral rim of the foramen magnum (Fig. 14). The lateral loose region promotes emergence of the C1 nerve root. In humans, an additional arcuate cluster of dense proatlas cells ventral to the notochord, likely an offshoot of the axial sclerotome and aptly called the hypochordal bow, gives rise to the bony anterior clival tubercle on the ventral surface of the basioccipital [66, 67] (Fig. 14).
First Three Cervical Somites (Somites 5–7)
Axial Sclerotomes
During resegmentation, the caudal half of somite 5 and the cranial half of somite 6 combine to produce the first cervical sclerotome; likewise, the second cervical sclerotome is made up of corresponding parts of somites 6 and 7. In the axial region of these sclerotomes destined to form vertebral centra, dense and loose zones appear in regular succession as in the lower cervical sclerotomes. The loose prevertebral zone of the first cervical sclerotome gives rise to the basal segment of the dens, and that of the second cervical sclerotome becomes the body of the axis (Fig. 14). Unlike in the more caudal sclerotomes, however, where the dense IBZ ultimately becomes the annulus and nucleus pulposus of an intervertebral disc, the dense zones in the first two cervical sclerotomes do not form true intervertebral discs and soon disappear [67]. Their intervertebral boundary mesenchyme gradually turns into the upper and lower dental synchondroses that ultimately cement the apical to the basal dens and the basal dens to the body of C2, respectively (Fig. 14).
Thus, after resegmentation, the human membranous axis consists of three median constituents that have been designated the apical dental segment from the caudal proatlas, the basal dental segment from the first cervical sclerotome, and the body of the axis from the second cervical sclerotome [6, 7, 37, 80, 84]. These three constituents chondrify simultaneously round 6 weeks gestation but remain segregated by the more cellular upper and lower dental synchondroses. Ossification of the cartilaginous axis occurs in three chronological waves (Fig. 16). The first wave appears as a single ossification centre within the axial body round 4 months gestation. The second wave begins at 6 months gestation as two separate ossification centres on each side of the basal dental segment [71, 76, 90, 100]. At birth, these two centres integrate and fuse, and the main component of the dens should at least have begun to show bony fusion with the axis body, even though a clear rarification may still be discernible at the lower synchondrosis till the 5th or 6th postnatal year (Figs. 16 and 17). Occasionally, the basal dens remains bifid (dens bicornis) when the third wave of ossification arrives within the apical dens round 3–5 years of age [6, 55, 76] (Figs. 16, 17, and 18). Ossification of the dental tip and bony fusion of the upper synchondrosis are not completed until adolescence [57, 58, 91, 96].
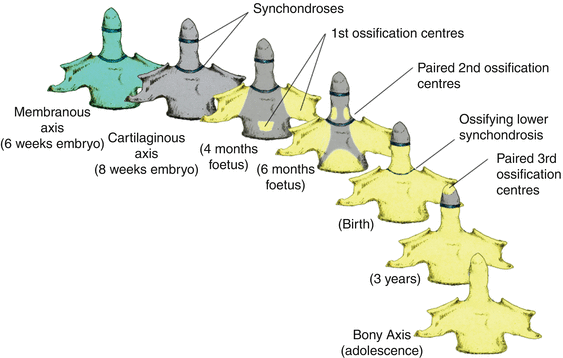
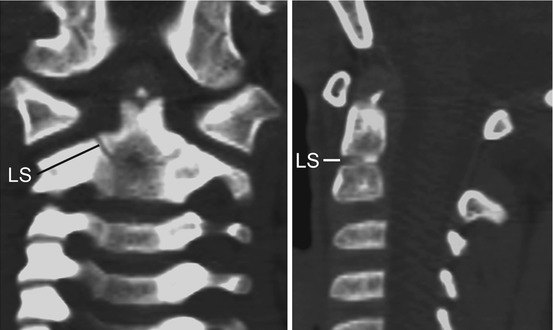
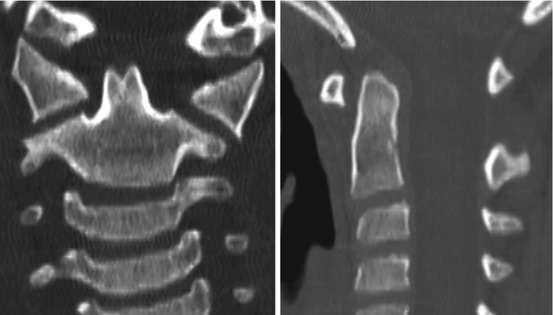

Fig. 16
The three developmental phases of the axis (C2) and the three waves of ossification. The primordia for the dens components are assembled during the membranous phase. Upper and lower dental synchondroses are shown as dense lines. First wave of ossification at 4th foetal month consists of bilateral centres for the neural arches and a single centre for the centrum. Second wave at 6th foetal month consists of bilateral ossification centres for the basal dental segment. At birth, the basal dental centres should have integrated in the midline and begun to be fused to the centrum. Third wave of C2 ossification occurs from 3 to 5 years postnatal life at the apical dental segment, which does not become fused to the basal dens till the 6th–9th year and fully formed during adolescence

Fig. 17
State of ossification of the dens of a 4-year-old child. The tip of the basal dental segment is bicornuate from bilateral secondary ossification centres. Small density above this represents early third wave of ossification within the apical dental segment. Note lower dental synchondrosis (LS) in the coronal and sagittal views

Fig. 18
Dens bicornis in a 7-year-old child. Lower synchondrosis has closed. Dens pivot is of normal height, suggesting the bifid tip is of the apical segment
Lastly, the apical ligament is almost certainly derived from the axial proatlas, and the alar and transverse atlantal ligaments are from the axial component of the first cervical sclerotome in association with the basal dental segments [67].
Lateral Sclerotomes
The lateral dense zone of the first cervical sclerotome develops into the posterior arch of the atlas, while the lateral dense zone of the second cervical sclerotome forms the arch of the axis. Their respective loose zones promote outgrowths of the second and third cervical nerves and segmental arteries. The hypochordal bow of the first cervical sclerotome ventral to the notochord subsequently forms the anterior arch of the atlas (Fig. 14, middle and right) [65–67, 84]. No definite hypochordal bows are seen caudal to this level, and equivalent cells in the lower segments appear to play no role in the formation of the vertebral column.
Genetic Control of CVJ Development
Hox Genes: The Control of Rostrocaudal Specification
Following primary segmentation, the determination of the positional identity of the prevertebral segments along the embryonic axis, which in turn ordains the regional developmental specifications of the vertebral phenotypes, is controlled by Hox genes. The mammalian Hox genes encode transcription factors used in regulating the establishment of the body plan. They contain the phylogenetically highly conserved homeobox domain [24, 43, 44]. In mouse and humans, there are 39 Hox genes distributed in four linkage clusters, Hox A, B, C and D, on four different chromosomes (chromosomes 6, 11, 15 and 2) (Fig. 19). The members of each cluster, designated by Arabic numerals, are also grouped vertically along the clusters because analogous members of each group are linked by common origin from a single ancestral gene. For example, Hox a– 4 , b– 4 , c– 4 , and d– 4 of the mouse, called paralogues, are held kin by all being the phylogenetic descendants of the much older Dfd gene of the Drosophila, and a–6, b–6, and c–6 are similarly related to the Antp gene of the Drosophila’s antennapedia complex (Fig. 19). Topographically, the lower numbered paralogues are located on the anterior 3′ axis of the chromosome, and the higher numbered ones are on its posterior 5′ locations (Fig. 19).
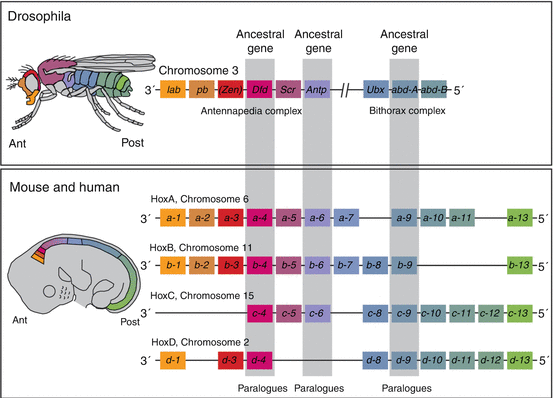

Fig. 19
Hox genes in mouse and human with their phylogenetic counterparts in Drosophila. Thirty-nine Hox genes are involved in the mouse and human vertebral column, found in four clusters of Hox A, B, C, and D on four chromosomes [2, 6, 11, 15], designated by Arabic numbers within each cluster and arranged as paralogues, so that the lower numbered Hox paralogues such as Hox a–1 and Hox d–1 are located on the anterior 3′ position of the chromosomes, and the higher numbered paralogues such as Hox a–13 and Hox d–13 are on the 5′ posterior position of the chromosomes. There is also temporal and structural colinearity with the embryonic axis so that the lower numbered paralogues are expressed earlier and more anterior on the embryonic axis than the higher numbered paralogues. (See colour match between genes and their expression domains on the embryonic axis.) Three sets of paralogues and their corresponding ancestral genes are designated by the grey bars
Hox genes are expressed in mesodermal and ectodermal cells along the body axis. Each gene has a characteristic and distinct anterior boundary of expression. A temporal and structural colinearity exists between the position of a gene in a cluster and its expression pattern. Thus, genes from the more anterior 3′ locations in the Hox clusters are expressed earlier and always occupy more anterior (cranial) expression domains than genes closer to the posterior 5′ location in the clusters (Fig. 19). For example, Hox a–3 has a more anterior expression domain than Hox a–9 and similarly between Hox d–4 and Hox d–10.
Functionally, only the anterior boundary of the Hox expression is important. Since multiple genes have the same anterior expression boundary along the prevertebral axis, each metameric segment can be identified by its own characteristic combination of Hox gene expression domains, i.e., its own Hox code (Fig. 20). A specific Hox code acts as a master switch in determining the exact positional identity of a mesodermal segment along the body axis, and via regional idiosyncrasies in development, the Hox code thus becomes translated into a specific vertebral anatomy [24, 34, 44].
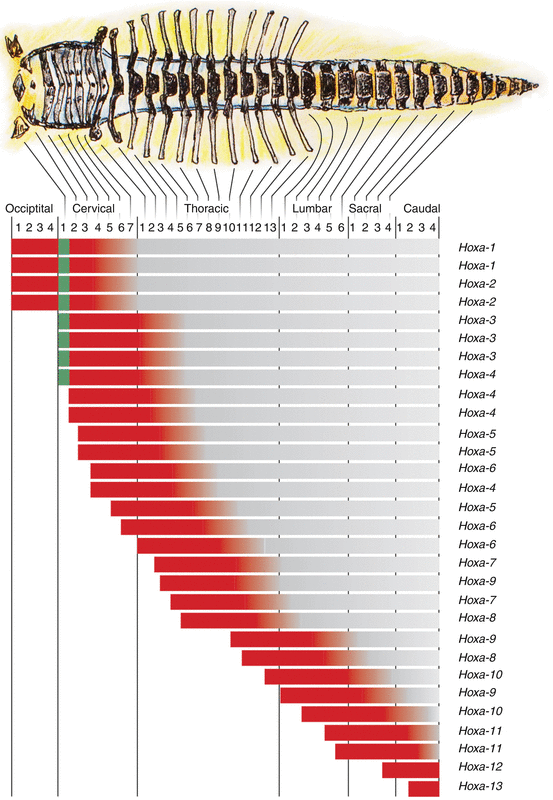

Fig. 20
Expression domains of Hox genes lined up with the mouse embryonic vertebral column. Only the anterior expression boundary (in red) is important, and since multiple genes have the same anterior expression boundary along the prevertebral axis, each prevertebral segment has its own combination of Hox gene expression domains (Hox code). For example, the Hox code for C1 is Hox a–1, b–1, a–2, b–2, a–3, b–3, d–3, and d–4 (designated by the vertical green bar)
The importance of the Hox code is illustrated by the severe abnormality in numbers and structures of vertebral segments when the code is altered by Hox gene mutations and teratogenic disturbance of Hox gene expressions. One example at the CVJ is the inactivation in mice of Hox d–3, an important component of the Hox code for the first cervical prevertebra. This results in a more caudal anterior boundary of Hox d–3 expression, shifting the transitional sclerotomal properties from C1 to C2 prevertebra and transforming the C1 prevertebra to a more anterior (i.e., occipital) identity, producing mutant mice with atlas assimilation to the basioccipital [13]. This is called anterior homeotic transformation (Fig. 21). Conversely, extension of an expression domain rostrally can transform prevertebral segments into a more caudal (posterior) identity, a phenomenon known as posterior homeotic transformation. This is exemplified in “gain-of-function” transgenic mutation of the murine Hox d–4 gene, also a component of the Hox code for the C1 prevertebra, such that its expression domain is forced to extend towards the occipital somites. The exoccipital region of the transgenic mutant shows no occipital condyles but instead ectopic neural arches resembling cervical neural arches, and the basioccipital is fused to the apical dens, “imitating” the behaviour of the atlas centrum [53]. In the human analogue of posterior homeotic transformation, the basion, from the O4 somite, “imitates” the C1 prevertebra by being detached from the upper clivus, and the anterior C1 arch, from the hypochordal bow of the C1 sclerotome, is fused to the apical dens as if C1 is trying to become C2 by acquiring a “true centrum” (Fig. 22). Thus, an altered interpretation of axial position cues in the developing occipital somites leads to an imposition of cervical-vertebral phenotype upon the occipital bones. These various observations suggest that Hox gene abnormality may underlie many malformations in the CVJ [17].
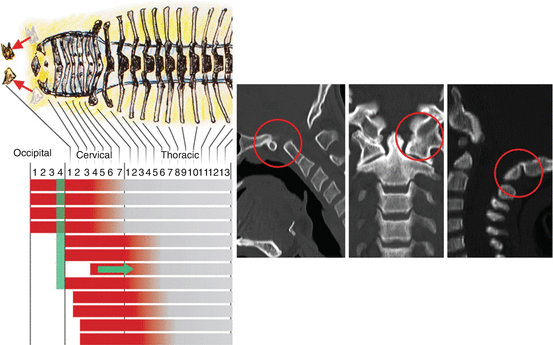
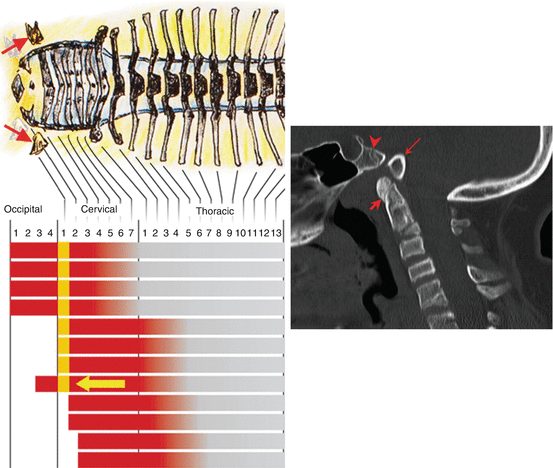

Fig. 21
Anterior homeotic transformation. Mutation of the Hox d–3 gene in the mouse causes a caudal recession of its anterior expression domain (green arrow), allowing the Hox code of the C1 segment (green bar) to resemble that of an occipital somite (Note green bar representing C1 Hox code has moved up to occipital sclerotome position). This renders the C1 sclerotome to “behave” like an occipital segment (symbolised in the figure by rostral movements of the C1 sclerotomes to a cranial position; see red arrows) and the anterior and posterior arches of C1 become fused to the basioccipital and exoccipital. Inset shows the human version of anterior homeotic transformation with assimilation of C1 to the occiput

Fig. 22
Posterior homeotic transformation. “Gain-of-function” mutation of the murine Hox d–4 gene causes its anterior expression domain to extend cranially (yellow arrow), allowing the Hox code for the O4 segment (yellow bar) to resemble that of the C1 and C2 sclerotomes (yellow bar moved to C1 Hox code position) and render the basiocciput to “imitate” the C1 segment (symbolised in the figure by caudal movement of the C1 sclerotomes; see red arrows on the drawing). The inset shows the human version of posterior homeotic transformation: the basion (long arrow) is detached from the upper clivus (arrow head), and the C1 anterior arch (short arrow) is fused to the apical dens as if C1 is trying to become C2 by acquiring a “true centrum”
Pax 1: The Resegmentation Gene
The Pax family of regulatory genes is implicated in sclerotomal resegmentation. Pax genes in vertebrates all contain the highly conserved DNA sequence called “paired-box”, in essence a paired domain with a partial or complete homeodomain. There are nine Pax genes in the mammalian family; all except Pax–1 and Pax–9 are involved in development of the central neuraxis. These two exceptions, especially Pax–1 (PAX–1 in the human homologue) [85, 97], control boundary formation between tissues by keeping two cell populations separate, presumably because the transcription factor encoded by Pax–1 differentially regulates cell surface molecules expressed by these two cell populations, thereby divergently influences their respective fates. This scenario of cellular partitioning is a necessary condition for resegmentation, and Pax–1 action at the future IBZ thus helps to demarcate the site and extent of sclerotomal segregation. The downstream target gene for Pax–1 is unknown but may involve cell adhesion molecules such as NCAM or cytotactin or molecules that promote cell-cell communication such as connexins [41, 42, 68, 85].
Pax–1 expression is detected very early in the pre-differentiated somites before their dorsoventral specification. Signals from the notochord and ventral floor plate of the neural tube, mediated by the SHH protein, induce the somite to divide into dermomyotome and the ventromedial sclerotome. This coincides with intense expression of Pax–1 ventrally within the sclerotomal field, suggesting that the earliest role of Pax–1 is mediating the dorsoventral specification of somites [48, 99].
After somitic differentiation, Pax–1 expression plays a more clearly segregating role within both the lateral and axial sclerotomes, where its timing and fluctuating levels coincide with crucial events of resegmentation; i.e., the opposite functions of partitioning and aggregation of adjacent sclerotomal clusters are matched to high and low Pax–1 expression, respectively. For example, during condensation of the axial sclerotome into the loose and dense halves, Pax–1 expression is weak within the loosely cellular prevertebrae but intense within the dense IBZ [85, 99]. Later, with chondrification of the prevertebra to form the homogeneous vertebral body, Pax–1 is further actively repressed in this location, but persists in high levels at the IBZ where partition of centra takes place, until formation of the intervertebral disc is well underway [99]. Pax–1 level is also enhanced during condensation of the lateral sclerotome to form the neural arch [23, 25, 98].
Conversely, normal fusion of certain adjacent sclerotomes takes place only when Pax–1 expression is turned off. At the CVJ of chick embryos, Pax–1 repression is timed exactly when the occipital sclerotomes fuse to form the basiocciput. The fusion of the two dens primordia with the axis body also coincides with the downregulation of the Pax–1 gene [99]. Ectopic Pax–1 expression disrupts normal assemblage of the dens-axis and basiocciput, producing mutants with mobile dental fragments and loosely beaded clivus [98]. Pax–1 is also highly expressed within the transitional zone between the proatlas and the first cervical sclerotome; it may thus also play a role in the separation of the head from the trunk.
Murine Pax–1 mutants undulated show multiple fusion of vertebral bodies and fusion of the dens with the anterior atlantal arch [98]. Extreme cases of complete fusion of the basiocciput-prevertebral column, literally from the clivus to the thoracolumbar spine, have been seen in human spondylocostal dysostosis (Fig. 23). These and the milder form of Klippel-Feil syndrome have been postulated to reflect severe PAX–1 loss-of-function mutations [85, 86]. It is therefore conceivable that hyper- and hyposegmentation defects in human may be explained by over- and under-expression of PAX–1 during vertebral development.
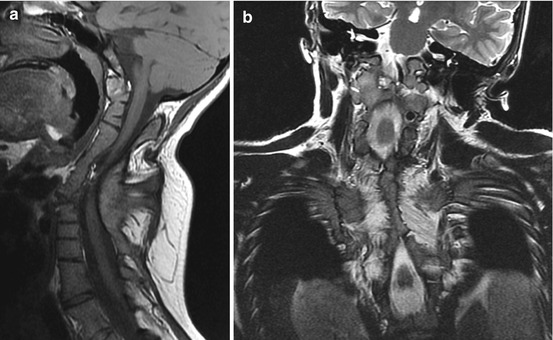

Fig. 23
Fourteen-year-old girl with spondylocostal dysostosis. (a) Sagittal MRI shows fusion of the clivus to the entire cervical and upper half of the thoracic spine. C1 and C2 are no longer identifiable by any of their unique features but had become part of a homogeneous rigid column. (b) Coronal MRI shows bilateral extensive fusion of the ribs, worse on the right thorax where almost all 12 ribs are fused
Classification of Bony Malformations of the CVJ
The large and seemingly disenfranchised rabble of bony malformations at the CVJ can now be grouped under the orderly logics of embryogenesis (Table 1). The first subdivision is between malformations of the central pillar and those of the surrounding rings, respectively due to a disturbance of the axial and lateral components of the relevant resegmented sclerotomes. Between the two main subclasses of central pillar malformations, the various types of odontoid dysplasias are better known because the odontoid pivot functions as the main axis for almost all motion planes at the CVJ, and its anomalous development severely disrupts the governance of safe motility of the craniocervical hinge. On the other hand, dysgenesis of the basioccipital block profoundly alters both the position and orientation of the odontoid and can lead to no less lethal impingement of the brainstem.
Within the other subclass of malformations of the surrounding rings, the teratogenic insult may be symmetrical or asymmetrical causing bilateral or unilateral dysplasia, involve the hypochordal bow and the lateral sclerotome individually or together, or affect the proatlas and the C1 resegmented sclerotome individually or simultaneously. Also, because the hypochordal bow may in fact be an early offshoot of the axial sclerotome from the same somite (or half-somite) and share sympathetic developmental fates, dysplasias of the anterior atlantal arch are not infrequently associated with centrum anomalies and together they result in extreme clinical instability.
Our knowledge of the genetic control of the embryology of the region is admittedly limited, but it is assumed that the known “master” transcription genes such as members of the Hox and Pax families each controls a crop of unknown regulator genes, and they, in turn, influence their own arbour of downstream progenitor genes. Mutation in any member gene within this enormous but interlinking tree, particularly higher up in the hierarchy, will likely affect multiple component phenotypes, and the permutations of associated CVJ anomalies may be asymptotic. The catalogue of anomalies in the present classification is therefore expected to expand or amend with future discoveries.
Disturbance of the Axial Component of Occipital Sclerotomes, Proatlas, and C1 and C2 Sclerotomes: Anomalies of the Central Pivot
There are two classes of central pivot anomalies: one concerns the dens-axis complex and includes the various forms of odontoid dysgenesis; the other afflicts primarily the basiocciput leading to an abnormal relationship between the odontoid and the skull base, comprising such entities as bifid clivus, platybasia, basilar kyphosis, basilar impression and a retroflexed dens.
Odontoid Dysgeneses
The making of any part of the vertebral column requires the successful completion of three developmental phases: First, the mesodermal primordium has to be properly formed and, in some cases, assembled during the membranous phase; second, the mesodermal primordium undergoes chondrification in the cartilaginous phase; and finally, in the osseous phase, ossification takes place within the cartilaginous mould to complete the end product. In the case of the dens-axis, there is a fourth phase which involves bony fusion of the upper and lower dental synchondroses (Fig. 16).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


