Primitive aortic arches develop in regular order from cephalic to caudal. There are two distinct phases in the development of the aortic arches: (1) the branchial phase, in which the vessels approximate to the branchial pattern of lower vertebrates, and (2) the postbranchial phase, during which the branchial pattern is being replaced by the adult arterial arrangement. During the branchial phase, the blood stream from the heart to the dorsal aorta on each side makes use of the following arches in succession: (a) first arch; (b) first and second arches; (c) second and third arches; (d) third and fourth arches; and (e) third, fourth, and pulmonary arches. During weeks 6–8, the aortic-arch pattern is transformed to the final fetal arterial arrangement (Fig. 2). The conversion of the early embryonic circulatory system to the adult configuration involves the following steps: (1) degeneration of parts of some primitive vessels, (2) hypertrophy of some primitive vessels’ parts, (c) anastomosis and/or fusion of some primitive vessels, (3) separation of one primitive vessel into two, (4) loss of connection between some primitive vessels, and (5) formation of new vessels [20].
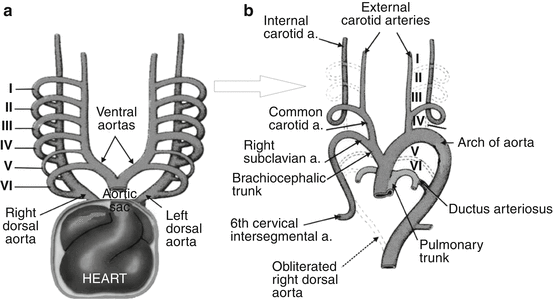

Fig. 2
(a, b) Modified scheme [131] of six (I–VI) primitive aortic arches during weeks 4–5 (a) and final arterial arrangement during weeks 6–8 of gestation (b)
The first primitive aortic arch largely disappears but the remaining parts form maxillary arteries and may also contribute to the formation of the external carotid arteries. The dorsal part of the second primitive aortic arch persists and forms the stem of the hyoid and stapedial arteries. Proximal parts of the third primitive aortic arch form the common carotid arteries; the distal part of the third pair of aortic arches joins with corresponding dorsal aorta to form the internal carotid arteries (ICAs). The left fourth aortic arch forms part of the arch of the aorta, whereas the right fourth aortic arch becomes the proximal part of the right SA [131]; the distal part of the right subclavian SA forms from the right dorsal aorta and right sixth cervical intersegmental artery (CIA). The left SA is formed from the left sixth CIA [83]. The primitive SA can first be recognized in embryos at the stage when the fourth arches are present but the pulmonary arches are not complete, yet [11].
Caudally to the aortic arches, the paired dorsal aortas merge to form a single descending aorta. Paired ventral aortas receive blood from the truncus arteriosus. It is worthy of note that no ventral aortas occur in the human embryo [11].
The aortic arch has a composite origin as it develops from the aortic sac, left fourth aortic arch, and part of the left dorsal aorta. The dorsal aorta degenerates between the third and fourth aortic arches. Failure of the normal caudal migration of the fourth aortic arch in the growing embryo from the occipital level in the thoracic cage at the 7th week of gestation has also been invoked as a cause of cervical ectopic of the aortic arch, as happened in a 3½-year-old boy and an adolescent [27].
Embryology of the Vertebrobasilar System
During the 4th week of gestation, 42 somites (4 occipital, 8 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 8–10 coccygeal pairs) are formed. Each somite differentiates into an outer dermatome and inner myotome and a medial sclerotome. Each dorsal aorta gives off about 30 or more intersegmental arteries that pass dorsally between somites. Segmental arteries send ventral and dorsal branches to the trunk wall. The sclerotomes are ventral–medial in their location and will form the vertebral bodies. The first four sclerotomes essentially fuse to form the occipital bone and posterior portions of the foramen magnum [65].
The serial cervical parts in the human embryo are eight nerves (and somites), seven vertebrae, and six CIAs [83]. These CIAs extend from the corresponding dorsal aorta toward the anterolateral aspects of the developing vertebrae, where they give rise to dorsal branches that pass between the vertebral transverse processes toward posterior vertebral muscles. The tunica adventitia of the large arteries of the neck became more evident than at 15 weeks [68].
At the 7–12 mm (5th to 6th week), the VA is formed as a longitudinal canal dorsal to the costal elements, interconnecting the dorsal branches of the CIAs from the first to the seventh [52], i.e., between the PIA and proximal six CIAs [83]. The trunk of the VA develops from the three parts: (1) cervical vertebral artery (a longitudinal anastomosis extending from the brachiosegmental to the suboccipital artery), which is subdivided into prevertebral and transversal parts; (2) atlantic part is portion of a vessel including the transversal spinal branch and the suboccipital artery; and (3) subarachnoidal part—a metencephalic longitudinal anastomosis in the direction of the caudal branch of ICA [52].
It is well known that anomalous origins of the VA arise from aberrant anastomosis at any time during the embryonic development of the aortic arch and/or cervical intersegmental arteries [114, 115].
In the hindbrain area, paired, separate, longitudinal neural plexus, as a precursor of the basilar artery (BA), appears at the 5- to 6-mm crown–rump length of the embryo (29-day). At this stage, the caudal branch of the ICA communicates with the ipsilateral longitudinal neural artery, which later becomes the definitive posterior communicating artery. Blood reaches them from dorsal aortas and ICAs via some primitive vessels—the trigeminal (PTA), otic (POA), hypoglossal (PHA), and proatlantal intersegmental (PIA) arteries. Bilaterally, the longitudinal neural plexus is supplied cranially by the PTA and caudally by the PIA [119, 121]. The caudal supply is also supported by input from the POA at the level of the VIIIth cranial nerve and from the PHA at the level of the XIIth cranial nerve [118, 120]. The longitudinal neural arteries have begun to form prominent anastomoses across the midline of the hindbrain or, rarely, they can completely persist, as in a 3-year-old boy [38].
Aplasia of the Vertebral Artery
Unilateral or bilateral aplasia of the VA in fetal and/or postnatal period can cause by persistence of carotid-vertebrobasilar anastomosis or it can to be a “satellite” of some syndrome or disease.
Khodadad [47], in his study of persistent PHA (PPHA) based on 50 dissected fetal brains, described that in most of the specimens, one of the VAs was absent while the second one was hypoplastic or, rarely, normal. Absence of the both VAs in the presence of the left PPHA was showed in a 14-day-old male baby with Arnold–Chiari malformation [36]. There were descriptions of absence of the both VAs in the presence of bilateral persistent PIA (PPIA) in a 4-year-old girl [78] and in a 6-year-old girl [89]. There was the absence of the left VA in the presence of the ipsilateral PPIA in a 3-day-old male neonate and in a 14-month-old girl [89] and in a 6-year-old boy [1]. Left vertebral angiogram showing retrograde flow in the persistent PTA (PPTA) indicated the absence of the ipsilateral VA in a 17-year-old girl [79]. A rare case of a “segmental aplasia” of the VA, the termination of hypoplastic right VA at the atlas level in the presence of the left PPTA, was also reported [44].
It was reported an absence of the right VA in a 4-year-old girl with PHACES syndrome and moyamoya vasculopathy [37], as well as an agenesis of the vertebrobasilar system and the ICA on both sides in a possible PHACE syndrome of a 14-year-old girl [126]. Investigation with Doppler ultrasound followed by arch aortography proved absence of the left VA in a 14-year-old boy with Takayasu’s disease [127].
Histology
The wall of the cerebral arteries of a 7-week-old embryo was composed of a single layer of endothelial cells. In a 9-week-old embryo, the walls consisted of an inner tunica intima, a central tunica media, and an outer tunica adventitia. At 12 weeks of gestation, two or three layers of smooth muscle cells had been added to the muscle coat of the media. In the embryos of more than 18 weeks old, the medial defect was not observed at the lateral angle of their branches; defects were observed at the lateral angle of the distal segments of these branches. The cerebral arteries of the embryos of more than 20 weeks old exhibited configurations similar to those of adult ones [29].
In addition to highlighting the striking difference between intracranial and extracranial arteries, data giving by Napoli et al. [71] indicated that brain arteries were less susceptible to hypercholesterolemia than extracranial ones in human fetuses and prematurely newborns (6.2 months, in average).
Extracranial Parts of the Vertebral Artery
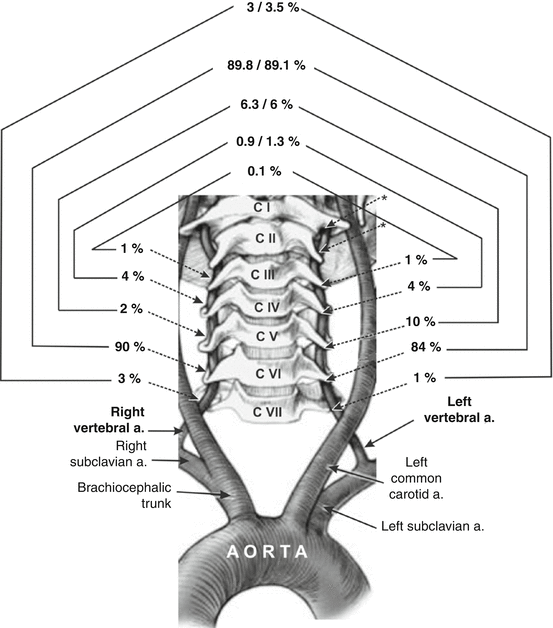
The VA, usually the first ascending branch of the SA, bilaterally courses through the neck (prevertebral, cervical, and atlantic parts) and through the foramen magnum and enters in the posterior cranial fossa (intracranial part), where it joins with the second one forming the BA. Incidence of an aortic origin of the left VA is on the second place among different patterns origin of the both VAs [4, 81, 114, 115].
Prevertebral Part
The prevertebral (V1) part of the VA extends from its origin to the point of entry into the foramen transversarium of the cervical vertebra, usually CVI.
Some authors stressed that an anomalous origin of the VA occurs mostly on one side, usually on the left [21]. In a study of 100 human fetuses [114], aged from 18 to 26 weeks of gestation and of both gender, the right and left VAs, as single vessels, originated directly from the superior or posterior wall of the SA in 94 and 83 % of cases, respectively (Figs. 3 and 4). In a study of 30 patients aged 19 years and older, the origin of the VAs was always from corresponding SA [21].
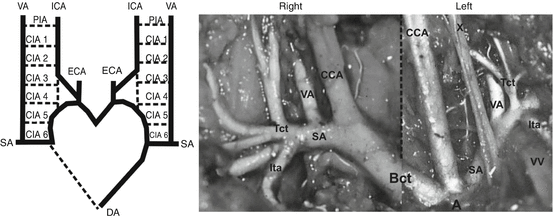
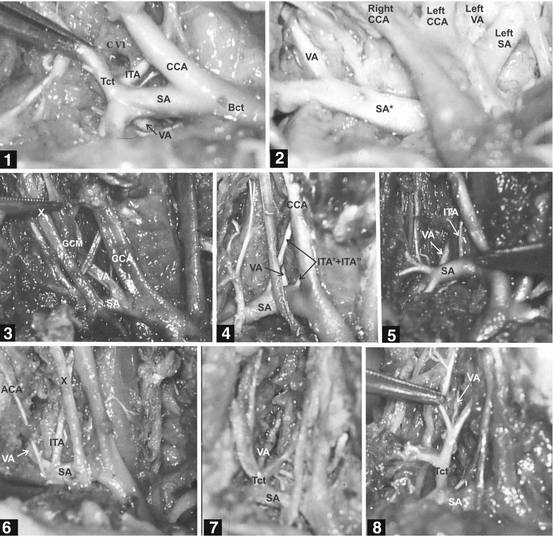

Fig. 3
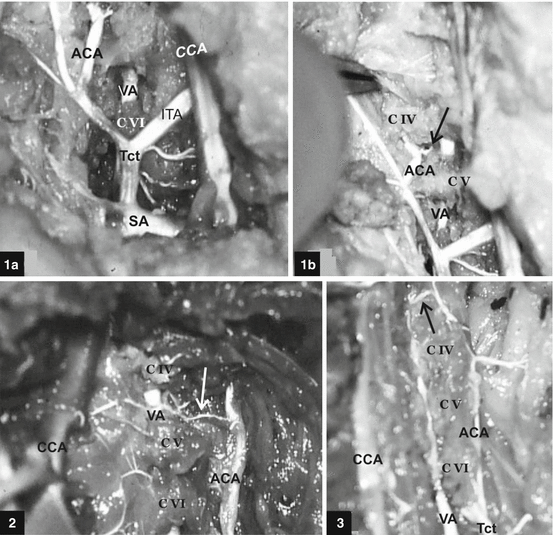
Modified scheme and photo of the vertebral artery (VA) origin on both sides in the human fetus. Scheme: Normal embryonic origin of the VA from the sixth cervical intersegmental artery [56, 83]. DA dorsal aorta, SA subclavian artery, CIA 1–6 six cervical intersegmental arteries, PIA primitive proatlantal intersegmental artery, ICA internal carotid artery, ECA external carotid artery. Photo: Origin of the VA from a superior wall of the SA, medially to the thyrocervical trunk on both sides [114]. A arch of the aorta, CCA common carotid artery, Bct brachiocephalic trunk, Tct thyrocervical trunk, Ita internal thoracic artery, VV vertebral vein, X vagus nerve

Fig. 4
Different modes of subclavian origin and relation of the right vertebral artery (VA) in human fetuses [114]. Case 1: Right VA originates from the posterior wall of the subclavian artery (SA) and penetrates CVII foramen transversarium. Case 2: Right VA originates from the superior wall of the arteria subclavia dextra lusoria (SA*). Case 3: Right VA originates from the superior wall of the normal right SA, just laterally to the bifurcation of the brachiocephalic trunk, and courses through the middle cervical ganglion. Case 4: Right VA originates from the posterior wall of the ipsilateral SA and courses between the two inferior thyroid arteries. Case 5: Right VA originates from the posterior wall of the ipsilateral SA and courses between the inferior thyroid artery and a “cervical” trunk. Case 6: A common origin of the right VA and inferior thyroid artery, just medially to the ascending cervical artery. Case 7: Right VA of normal caliber originates from the thyrocervical trunk. Case 8: Right VA of extreme hypoplastic caliber originates from the thyrocervical trunk. Bct brachiocephalic trunk, CCA common carotid artery, Ttc thyrocervical trunk, ITA inferior thyroid artery, ACA ascending cervical artery, X vagus nerve
The VA orifice on the right SA was most frequently between 20 and 30 mm, at the junction of the posterior and superior surfaces of the SA in adults aged 20 years and older [4]. In relation to the thyrocervical trunk, the VA was usually located 5 mm medially, as cited in the paper of Sartor et al. [92], or 10 mm before, sometimes at and less frequently immediately beyond the thyrocervical trunk, as noted Argenson et al. [4]. An origin of the VA at the level, or laterally from the thyrocervical trunk, Jung and Kehr [42] and Zlotnik and Gitkina [132] defined as a lateral displacement of the VA orifice, when scalene muscles can compress the VA. Incidences of 0.58 % was noted in the literature for a common stem of the VA and thyrocervical trunk originating from the SA; the incidences of 0.64 and 0.13 % were noted for a common stem of the right and left VA and inferior thyroid artery, respectively [92].
On the tomographic image demonstrated by Borenstein et al. [9], it was evidenced that the normal right SA lies 3 mm above the level of the aortic arch and 14.8 mm above the level of the four-chamber view in the 20-week fetus. The values of an external diameter of the left SA ranged from 0.68 to 2.89 mm for the 4-month and 9-month fetuses, respectively [104]. Average external diameters of the right (left) SA were 3.6 (3.4), 4.8 (4.7), and 6.4 (5.9) mm of 19 mature newborn babies, infants, and children to the 4th year [59]. An average depth from the skin to the VAs in infants of about 20 months old was 15.1 ± 3.3 mm [111].
It is fact that VA is usually the branch of the SA; however, it is well known that SA could not be the branch of the brachiocephalic trunk on the right side and/or last branch of the aortic arch. There are a lot of these examples. One of these is the arteria subclavia dextra lusoria (ASDL)—a last branch of the aortic arch (beyond the left SA). It was founded in 1 of 100 human fetuses (see case 2 at Fig. 4) [114, 117], as well as in 1 of 103 human fetuses [31], and in 1.5 % of chromosomally normal fetuses [9]. However, it was found only in 1 of 173 fetuses [107]. The ASDL was also founded in a 7-week-old infant with a large facial hemangioma [106], as well as in an 8-year-old girl and in a 9-year-old boy with frequent respiratory infections, and in a 10-year-old boy with epilepsy [5]. With regard to the VA, it was the branch of the ASDL in previous fetal and infant cases, as well as in 84.3 % of patients with proved ASDL, aged from 2 to 16 years [109]. However Loukas et al. [57] found ASDL simultaneous with a common trunk of the right VA, and left thyroid ima artery arose from the aortic arch between the right and left CCAs during a medico-legal autopsy of a 12-year-old boy.
Two cases (a 6-month-old boy and 2-year-old girl) with tetralogy of Fallot and a right aortic arch were unusual because in each of them an atretic left SA arose from the left pulmonary artery; however, the SA was pulsate distally in both cases, where the vertebral, internal thoracic, and thyrocervical vessels arose [53].
Three infants (2-day-old boys and girl) with aortic arch anomalies had an origin of both SAs from descending aorta; however the beginning of the VA was from the corresponding SA [97].
An anomalous vessel of irregular shape, up to 10 mm in diameter and 4 cm in length, arising from the VA at the level of the CVI transverse process and terminating in the subclavian vein in a 4.5-year-old boy was defined as a rudimentary accessory SA [50].
The average diameter of the aortic arch ranged from 1.08 mm in 14–15 weeks’ gestation [2] to 4.09 mm in 29–30 weeks’ gestation [31] and 4.79 mm in 36–38 weeks’ gestation [2]. In two studies of 100 and 200 human fetuses in Serbia, the left VA, as a single vessel, originated from the aortic arch in 11 of 100 [114] and in 18 of 200 cases [115]; a segmental V1 duplication was found in 4 and 2 % of fetal cases, respectively. The VA was localized on the aortic arch between the left CCA and SA in 17 of 18 cases (Figs. 5 and 6) and beyond to the left SA in one case (see case 5 at Fig. 6). In a study of Colombian fetuses, a variation of the arch with four branches—brachiocephalic trunk, left CCA, left VA, and left SA—was founded in 15.7 % of aortic arches [81]. Opposite to previous findings, the left VA sprang directly from the aortic arch between the left CCA and left SA in 7/103 (6.8 %) human fetuses aged from 14 to 30 weeks of gestation [31], as well as in 7 of 131 (5.34 %) human fetuses aged from 15 to 34 weeks [103]. Similar incidence is proved in adults; a beginning of the left VA from the aortic arch arose between the origins of the left CCA, and SA was noted in 6/104 subjects (54 Black African and 50 European subjects) aged 20 years and older, respectively [4]. Eighty-six (4.2 %) in series of 2,033 patients (between 3 months and 94 years) had a left VA originating directly from the aortic arch proximal to the left SA, except the one case where it was distal to the left SA [70]. However, digital subtraction angiographies belonged to 447 male and 186 female Greek patients aged 19 years and older revealed the left VA on the aortic arch between the left CCA and left SA in 0.79 % of cases [72].
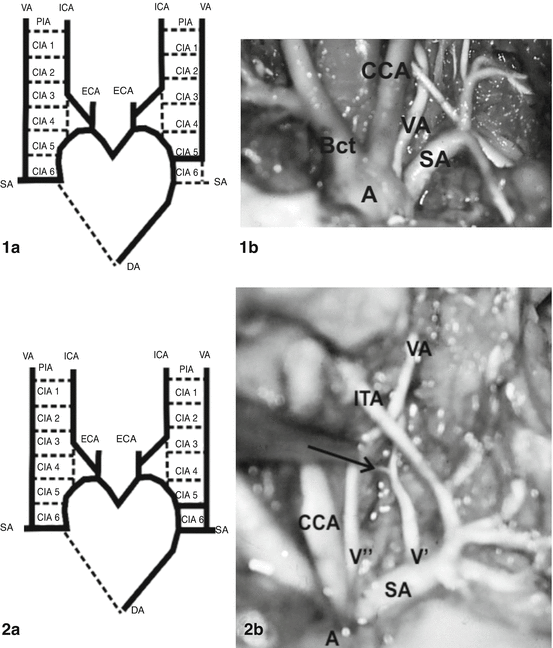
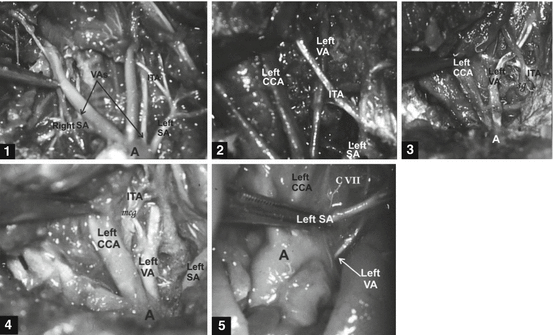

Fig. 5
Aortic origin of the left vertebral artery (VA) in two human fetuses [114, 115]. Case 1a: Modified scheme of embryonic origin of the VAs [56]. The left VA originating from the aortic arch is caused by a persistence of the 5th cervical intersegmental artery (CIA). DA dorsal aorta, ICA internal carotid artery, ECA external carotid artery, PIA primitive proatlantal intersegmental artery. Case 1b: Left VA, as a single vessel of aortic (A) origin (between left CCA, common carotid artery and left SA, subclavian artery). Bct brachiocephalic trunk. Case 2a: Modified scheme of embryonic origin of the VAs [56]. Segmental duplication of the left VA is caused by a persistence of the 5th and 6th cervical intersegmental arteries (CIAs). Case 2b: Dual vessels of aortic (V″) and subclavian (V′) origin; these vessels course through V1 part behind the ipsilateral inferior thyroid artery (ITA). A collateral branch (→) from V′ vessel is presented

Fig. 6
Five cases of the aortic origin of the left vertebral artery (VA) in human fetuses [114, 115]. Cases 1–2: Left VAs of aortic (A) origin (between the left CCA, common carotid artery, and the left SA, subclavian artery) pass behind the left inferior thyroid artery (ITA) in both cases. Case 3: Left VA of aortic origin (between the left CCA and left SA) crosses anterior wall of the ipsilateral ITA at the level of the stellate ganglion (sg). Case 4: Left VA of aortic origin (between the left CCA and left SA) is vascular source of the left ITA; it passes through the middle cervical ganglion (mcg). Case 5: Left VA of aortic origin (behind to the left SA) passes in front of the CVII vertebra to the CVI foramen transversarium [115]
Literature case reports showed an aortic origin of the left VA (between the left CCA and left SA) simultaneous with the ipsilateral aplasia of ICA in a 21-year-old male with subdural hematoma [128], as well as the beginning of the left VA from the aortic arch beyond the left SA in a 6-year-old boy with small perimembranous ventricular septal defect [102].
Oppido et al. [80] disclosed a right aortic arch with four separate branches—right CCA, right VA, right SA, and aberrant left SA—in a newborn girl with tetralogy of Fallot and DiGeorge syndrome. Felsont and Strife [27] presented an unusual literature case of the left VA origin from the cervical aortic arch (between the left CCA and left SA) in a 3.5-year-old boy.
Both VAs originated separately from the aortic arch, which was narrow from the brachiocephalic trunk to the origin of the patent ductus arteriosus in a 6-day-old male infant [62]. Both VAs showed an anomalous origin in a 13-year-old female patient with ASDL: the right VA arose from the right CCA, whereas the left VA arose from the aortic arch [109]. The aortic origin of both VAs beyond the left SA origin was also shown in a 20-year-old male patient with a spondyloepiphyseal dysplasia [33]. Multidetector CT angiography performed by Oguz et al. [76] in a 7-year-old boy with symptoms of gastroesophageal reflux identified the left VA with two separate origins, from the left CCA and aberrant left SA (beginning from right-sided aortic arch). Segmental duplication of the right VA originating from the ipsilateral SA was proved in one human fetus [114, 116], as well as in an 18-year-old epileptic female [6].
The V1 part of the VA is surrounded by the cervical sympathetic plexus and by two vertebral veins [113]. The diameter of the vertebral vein in adults is variable but usually three times smaller than the VA [4]; however, this difference was not showed at V1 part of fetuses (Fig. 7) [114]. It was noted that 20 % of the cranial outflow of 16 normal babies normally passed down the vertebral veins [18]. In some instances an arteriovenous fistula could be found just below the transverse process of the CVI vertebra, as in a 20-year-old man [98].
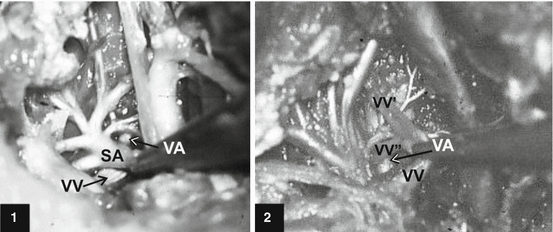

Fig. 7
Single (case 1) and dual (case 2) right vertebral veins (VV) at the exit from the transversal canal and their relation with the ipsilateral vertebral artery (VA) of the subclavian (SA) origin in human fetuses [114]
Of the vessels examined, 60 % of VAs followed a relatively straight course from their origin to their entry into the foramen transversarium [21]. A tortuosity of the VA in fetal status was found only in one case of its origin from the left SA beginning on the aortic arch, whereas of 42 infants aging from 2.5 to 14 years, 15 infants had tortuosity of the extracranial VA, while 5 infants had bilateral asymmetric underdevelopment of the VA with tortuosity on the right side [125]. The tortuosity and/or compression of the VA can be congenital, muscular (due to compression by the longus colli and scalene), or because of advancing years [113]. So, Jung and Kehr [42] performed a section of the right scalene in a 16-year-old girl with vertigo symptoms in the aim of the VA decompression in the transversal canal from CVI to CIV vertebrae.
From its origin from the SA, the VA traverses the subclavian triangle, lateral to the longus colli muscle and medial to the scalenus anterior muscle to penetrate the foramen transversarium of CVI vertebra. An anomalous vessel of irregular shape, up to 10 mm in diameter and 4 cm in length, arising from the VA at the level of the CVI transverse process and terminating in the subclavian vein, in a 4.5-year-old boy was defined as a rudimentary accessory SA [50]. Two vertebral vessels from the left CCA and aberrant left SA in a 7-year-old boy united at CVI vertebral level to continue as the single VA and entered the foramen transversarium at the same level [76].
The VA is vulnerable at the V1 part, where it is traversed by fascial bands and skeletal muscles [30]. In relation to the inferior thyroid artery (ITA), the fetal VA was located behind (see Figs. 3 and 5; case 3 at Fig. 4; cases 1–2 at Fig. 5) or in front of it (Fig. 8); however, a rule was not found [114].
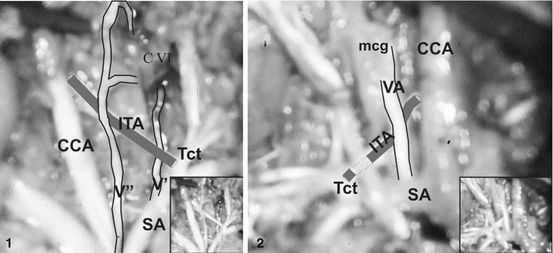

Fig. 8
Relationships between the vertebral (VA) and inferior thyroid (ITA) arteries [114]. Case 1: Vertebral vessel (V″) of aortic origin courses in front of the ipsilateral ITA; vertebral vessel (V′) of subclavian origin courses behind the ITA. Insert: original photo. CCA common carotid artery, SA subclavian artery, Tct thyrocervical trunk, CVI sixth cervical vertebra. Case 2: Right VA originating from the superior wall of the SA courses in front of the ipsilateral ITA, and through the middle cervical ganglion (mcg). Insert: original photo
Incidences of VA penetration of the foramen transversarium of cervical vertebrae at different levels in prenatal and postnatal status are presented at Fig. 9. Literature data indicated that in slightly over 10 % of subjects, the VA does not enter the foramen transversarium at CVI but at other levels, from CVII to CII, as well as that the entrance is symmetrical in 85 % of cases and asymmetrical in 15 % [32]. Some authors noted that VA penetration of the foramen transversarium at high cervical level was in relation with aortic origin of the VA. However, the left VA, as the last branch of the aortic arch in one fetus, penetrated the foramen transversarium of the ipsilateral CVI vertebra (see case 5 at Fig. 6). In addition, the right VA, as a last branch of the aortic arch distal to the origin of the left VA, coursed behind the trachea and the esophagus and entered the ipsilateral foramen transversarium of the CVII vertebra [33].
Histology
The wall of the VA, like all human blood vessels, consists of three layers: tunica intima, tunica media, and tunica adventitia; it is a muscular (distributing) artery [43, 67].
The tunica intima of the VA in the newborn is thin, plane, whereas the internal elastic lamina is thicken and uneven. The tunica media consists of single elastic fibers, which are embedded between muscle fibers; its thickness is about 70 μm. The tunica adventitia consists of collagen and single elastic fibers; its thickness is about 100 μm. There is an increase of elastic fibers in the tunica media and adventitia in a 5-month-old infant; the thickness of the media and adventitia is about 100 and 200 μm, respectively. The number of elastic and collagen fibers increases in tunica media until the 15th year of life. The thickness of the tunica media and adventitia is 220 and 200 μm in the 16th year [48].
The adventitia of the VA is fixed at the transverse process of the CVI vertebra, and consequently this anatomical feature prevents injury to the VA from various neck movements [77].
Cervical Part
The cervical (V2) part comprises the segment of the VA that usually extends from the CVI to the CII foramen transversarium.
During the fetal–neonatal period, the cervical vertebrae (CIII–CVI) consist of three independent osseous components: a centrum and two neural arches which are joined to each other by a hyaline cartilage. The CII vertebra (axis) consists of four osseous components: a centrum, two neural arches, and dens. The CVII vertebra may be described as a transitional one; superior articular facets are seen to extend transversely to the top of the transverse processes [14]. The posterior aspect of the foramen transversarium is formed from the neural process; the anterior portion is formed by a vestigial costal element that fuses to the vertebral body, encasing the VA within the foramen [75]. Since a longitudinal anastomosis runs ventral to the 7th costal element in the human embryo, the VA usually does not pass through the foramen transversarium in the CVII vertebra [7]. The VAs ran vertically in the vertebral grooves (later foramina) in four human embryos (45, 50, 53, and 58 days) and in a 77-day-old fetus [19]. At 8 weeks of gestation, the cervical nerves and ventral branches of C2 to C6 lay laterally to the VA; the C4 to C7 nerves give twigs to the VA [75].
If the vascular system is a factor of the canal form through transverse processes of cervical vertebrae during embryonic development, vascular system tolerates an opposite effect and secondary changes caused by deformation of canal walls [22]. Since the CIA is instrumental in the formation of the definitive vertebral body during the stage of resegmentation, any abnormal distribution of the CIAs may induce malformation [108]. In addition, abnormalities in the fusion of CIAs can also contribute to some tortuosity of the VA [21, 113]. Tortuous VAs may have an eccentric position in an enlarged foramen transversarium; the area of this foramen occupied markedly in some patients aged 7 years and older was with a range of 8–85 % [91]. A straight course of the VA is found in 94 % of cadaveric specimens aged 16 years and older [45]. A dolicho left VA running in a short loop toward the midline was visible at angiographic film of an 18-year-old girl [6]. A fenestration of the left VA at the level of CV vertebra in a 19-year-old male was also discovered [49].
The posterior placement of the vertebral foramina in relation to the body of the vertebra increases vulnerability to VA compression by the subluxation of the apophyseal articulation [11]. The distance between the medial borders of the transversal foramina is the greatest at the level of C VI vertebra; it narrows as the VA ascends, and is the narrowest at the level of C III vertebra [82]. In the upper cervical spine, the VA is most vulnerable to compression and stretching, as well as forced lateral flexion, during head rotation. Veleanu (1974) has stressed that the transverse processes of the axis are not ossified in fetuses and newborns, and for this reason during a forced rotation or lateral flexion or extension of head and neck there is the possibility for the compression of the VA. James et al. [39], Pamphlett and Murray [84], and Mitchell [66] also supported the fact that an extreme extension or rotation of the head can occlude one or both VAs. One case involved a 7-year-old boy who sustained a left VA occlusion at the axis level after a chiropractic adjustment, whereas the second one involved a 21-month-old girl who suffered a thalamic stroke due to VA compression as a result of head turning [87]. A significant decrease in the VA blood flow velocities was found with both ipsilateral and contralateral rotation of the cervical spine in healthy adults aged 20 years and older; this significant difference was maintained in the male subjects but with contralateral rotation, only, in the female subjects [66].
An extraspinal system is highly developed in the cervical region, where the VA and the deep cervical and ascending cervical arteries form the most effective longitudinal anastomoses [17]. These anastomoses are proved in fetal cases (Fig. 10), too [114]. Anterior and posterior ascending arteries of the VA originating at the level of CIII vertebra ascend to the dens and meet superiorly to form an apical arcade [101]. This posterior branch of the VA in reality is the anterior meningeal artery or so-called C3 artery; it anastomoses with ascending pharyngeal collaterals laterally or with occipital or deep cervical artery posterior [55].