Chronic Kidney Disease
Denis F. Geary
The definition of chronic kidney disease (CKD) and its different stages are outlined in Table 477-1. The term chronic kidney disease has been introduced to ensure consistency of terminology in relation to patients with sustained renal disorders, and to replace the terms chronic renal insufficiency and chronic renal failure.1 The definition of CKD excludes patients younger than 2 years whose renal function improves markedly in the first 2 years of life; therefore, a glomerular filtration rate (GFR) < 90 mL/min/1.73 m2 in a 6-month-old child may not represent any abnormality of renal function.
 CAUSES OF CHRONIC KIDNEY DISEASE IN CHILDREN
CAUSES OF CHRONIC KIDNEY DISEASE IN CHILDREN
Causes of chronic kidney disease (CKD) in children do not vary substantially between disease registries from a variety of countries.2,3 In younger children, the most common causes are congenital abnormalities of the genitourinary system that are accompanied by vesicoureteric reflux or obstruction to urinary outflow leading to renal hypoplasia or dysplasia. The most common obstructive lesions are posterior urethral valves and prune belly syndrome, both of which only occur in boys (see Chapter 476). Renal cystic diseases, including multicystic kidneys, cystic renal dysplasia, juvenile nephronophthisis, and autosomal-recessive polycystic kidney disease, may cause significant loss of renal function during childhood (see Chapter 470). Glomerular diseases causing significant renal disease in early childhood rarely include congenital nephrotic syndrome, or more commonly, focal segmental glomerulosclerosis or hemolytic uremic syndrome (see Chapter 472). In teenage years, membranous nephropathy and membranoproliferative glomerulonephritis may be seen, and CKD may be seen in association with systemic lupus erythematosus.
 INCIDENCE AND PREVALENCE
INCIDENCE AND PREVALENCE
According to the US Renal Data Systems report, there were 1325 incident patients ages 0 to 19 years with end-stage renal disease or renal transplants treated in 2005, with a prevalent population, including renal transplant patients, of 7362.4 The prevalence is too low to provide accurate estimates of prevalence of each chronic kidney disease (CKD) stage. However, more detailed information is available in the Italian registry from 1990 to 2000, including 1197 children with a creatinine clearance < 75 mL/min/1.73 m2 body surface area (BSA) (predialysis) and age younger than 20 years at the time of registration. This registry reported a mean incidence of 12.1 cases per million (range 8.8–13.9), and the prevalence was 74.7 per million of the age-related population.3
It is clear that expanding the diagnosis of CKD to include patients with normal renal function but abnormal urinalysis or radiologic appearance (as recommended by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative [NKF KDOQI]) greatly increases both the incidence and prevalence data. However, many of these patients have very minor renal disease.
 COMPLICATIONS
COMPLICATIONS
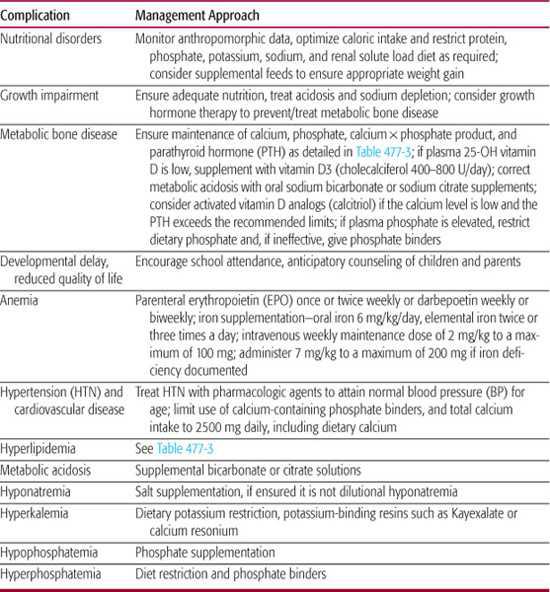
The complications that should be anticipated in children with chronic kidney disease (CKD) are listed in Table 477-2. Including stage 1 CKD, an overall complication rate of 70% hypertension, 37% anemia, 17% metabolic bone disease, and 12% growth failure has been observed.5 The frequency and severity of these complications of CKD increase as the stage of CKD increases. Consensus management recommendations for the complications of CKD have been provided by the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines and by groups of European expert panels, as summarized in this section.
Nutritional Disorders
Malnutrition in children with chronic kidney disease (CKD) is often accompanied by protein-energy wasting, which is characterized by decreased body stores of protein and energy fuels (fat mass).6 In CKD, additional factors that contribute to development of protein-energy wasting may include nonspecific inflammation, transient infections, chronic acidemia, resistance to insulin, growth hormone resistance, increased glucagon levels, hyperparathyroidism, and blood loss through dialysis or from frequent phlebotomy.6 Although the underlying cause of malnutrition in children with CKD is often multifactorial, it is extremely important that it be recognized and treated promptly. Nutritional assessment should include measurement of height, weight, body mass index (BMI; weight/height2), head circumference in infants, skinfold thickness, mid-arm circumference, serum albumin, cholesterol, and C-reactive protein (as a marker of nonspecific inflammation). No single one of these measures will define malnutrition, but in a child with CKD, where two or more are abnormal, attention should focus on his or her nutritional state (see Chapter 28). Nutritional support from a specialized dietitian is necessary to optimize caloric intake, which is the overriding goal of treatment for malnutrition in children with CKD.
Table 477-1. Stages of Chronic Kidney Disease
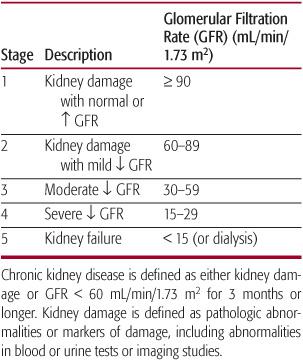
Table 477-2. Complications of Chronic Kidney Disease in Children
Achieving adequate caloric intake may be difficult. Children with CKD often have an impaired appetite, possibly associated with impaired taste and sometimes with nausea and vomiting. They may also require the restriction of specific dietary components, especially potassium and phosphate, and of the renal solute load. Polyuria may also inhibit intake of other calorically dense liquids in children with congenital renal disease that require increased water intake due to the inability of the kidney to concentrate urine. Thus, administration of a formula with a low renal solute load (low protein, sodium, and potassium) is often necessary. Breast milk can be used, but specialized infant formulas designed for children with CKD are often required. These have lower sodium, potassium, and phosphorus, as well as a lower renal solute load, than other infant and pediatric formulas. In North America, these include PM 60/40 and Good Start.
If additional caloric density is required, it is preferable to add modular commercial components rather than to simply concentrate the formula because concentration increases electrolyte and mineral content. These include a variety of protein powder (Beneprotein powder, Resource), carbohydrate (Polycose, Bene-calorie), and fat (vegetable oil, Microlipid) products. Specific adult “renal formulas” that are high calorie with low electrolytes and phosphorus are not recommended for use in children younger than 2 years due to their high osmolality and inappropriate mineral content, especially higher magnesium content. Due to the child’s poor appetite, and sometimes unpalatable formula and fluid requirements, it can be unrealistic to expect an infant or child to achieve adequate nutrient intake by mouth. In these circumstances, it may be necessary to provide enteral nutrition (nasogastric or gastrostomy) or parenteral nutrition. Approaches to the administration of enteral and enteral nutrition are discussed in Chapter 28. Percutaneous gastrostomy placement is contraindicated if peritoneal dialysis is likely.
Consultation with a specialized dietitian with expertise in the management of children with CKD is often necessary. Psychologic consultation may also be necessary to assure continued dietary compliance in older children where difficulty in compliance with the required restricted diet is common.
Growth Impairment
Impaired growth is a common complication of chronic kidney disease (CKD); it can have a profound psychologic impact on a child and adversely affect his or her quality of life. Population studies show increased mortality in children with CKD and severe growth delay.7 Children with CKD are approximately 1.5 standard deviations below normal for height, and this height deficit is greatest in the youngest children, despite improved efforts to sustain growth.2 About one third of all children with CKD and about one fifth of those with glomerular filtration rate > 50 mL/min/1.73 m2 have a height below the third percentile for age. The maximal deterioration in height seems to occur in the first several months of life or prior to referral to a pediatric nephrolo-gist; once appropriate treatment of their undernutrition and renal osteodystrophy ensues, a normal growth pattern is usually established until at least stage 5 CKD (dialysis) is reached. However, growth lost in these first months of life may be very difficult to restore.
The causes of growth delay in children with chronic kidney disease are numerous, and include malnutrition/protein-energy wasting, chronic acidosis, severe renal osteodystrophy, sodium depletion, and growth hormone (GH) resistance. Where possible, treatment should be directed to correction of these metabolic disturbances prior to introduction of GH.
GH is not deficient in children with chronic kidney disease, and in fact, plasma GH levels may be elevated. However, a number of abnormalities have been demonstrated in the steps required for activation for GH. These abnormal steps in the GH metabolic pathway include abnormal actions of GH directly on bone, defective conversion of GH to insulin-like growth factor 1 (IgF-1) in the liver, and accumulation of IGF-binding proteins in the serum, some of which interfere with the action or IgF-1 on the bone.
Randomized controlled trials show that growth hormone (GH) treatment can promote short-term growth in children with chronic kidney disease (CKD). Previous concerns about the potential adverse effects of GH therapy and the possibility of a diminishing effect with prolonged usage have not been substantiated by long-term usage studies.8 Occasional complications of GH use include pseudotumor cerebri, worsening renal osteodystrophy, and slipped capital femoral epiphysis, but the incidence of these is probably no different than children with CKD who are not treated with GH.9 Therefore, current consensus recommendations are that children with growth impairment and CKD, for whom no other cause is apparent, be treated with GH. Despite this recommendation, only about one in five children with a height less than the fifth percentile are prescribed GH.2
Metabolic Bone Disease
Metabolic bone disease affects many children with chronic kidney disease (CKD), and in its severe form deformities associated with rickets will impair growth. The exact cause of the bone abnormalities seen in children with CKD is not clear, but two pathogenetic mechanisms are recognized: (1) insufficiency of vitamin D from deficient activation of cholecalciferol to the active form of 1,25-dihydrocholecalciferol in the kidney or due to low cholecalciferol levels as a result of dietary insufficiency; and (2) retention of phosphate as renal function declines. Elevation of phosphate levels in plasma produces a reciprocal fall in plasma calcium values, which in turn stimulates parathyroid hormone (PTH) secretion; PTH then increases phosphate excretion by suppression of tubular phosphate reabsorption, thereby returning phosphate values to normal, but at the expense of persisting elevation of PTH values. These mechanisms are illustrated in Figure 477-1.
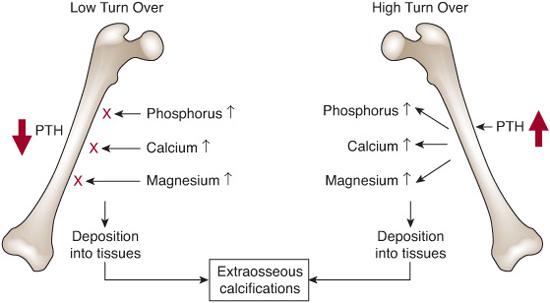
Over the past two decades, renal bone disease has been classified according to the findings on histomorphometry of tetracycline-labeled bone tissue. Hyperparathyroidism produces a state of high bone turnover with osteitis fibrosa and thickened irregularly shaped trabeculae with initially increased, but later reduced, bone volume; increased resorption of cortical bone also produces decrease in cortical bone volume.10 More recently, low turnover (adynamic) bone disease has been reported in almost one third of children, and may result from attempts to treat hyperparathyroidism with activated vitamin D compounds and calcium-containing phosphate binders.10
The effects of metabolic bone disease on the epiphysis are unique to children with chronic kidney disease, and may include growth failure, slipped epiphysis, and, as mentioned previously, rickets. The effects of low turnover bone disease, which leads to increased fractures in adults, are less clear in children but may adversely affect growth. As shown in Figure 477-2, both low and high turnover bone disease may impair mineralization of bone and contribute to calcification of vessels.
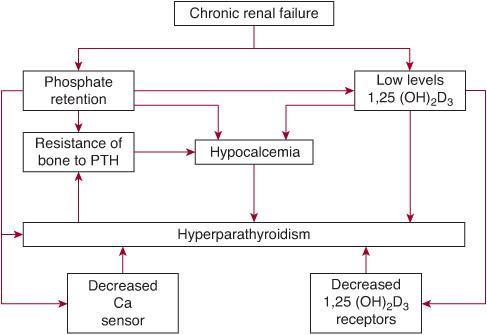
FIGURE 477-1. Schematic representation of the factors involved in the pathogenesis of secondary hyperparathyroidism. PTH, parathyroid hormone.
Because expertise is not available to evaluate bone histomorphometry in most pediatric hospitals, recognition of metabolic bone disease in chronic kidney disease relies on a number of surrogate markers, and imaging techniques. Historically, x-rays of the knees, wrist/hand, and hips have been used, and may demonstrate changes of rickets, or subperiosteal resorption due to hyperparathyroidism, but the sensitivity for detection of mild disease is poor. Also, radiologic techniques will not distinguish high turnover from low turnover states. Dual-energy x-ray absorptiometry (DXA) is of limited value to evaluate renal osteodystrophy because (1) it measures bone mineral density as total bone mass in a given area and may not detect structural alterations in trabecular and cortical bone density and architecture, (2) DXA does not distinguish the effects of PTH on cortical and trabecular bone, and (3) interpretation of results is confounded by impaired growth, and whether comparison should be with height or chronologic age as well as pubertal stage. Therefore, great reliance is placed on surrogate biochemical markers to detect and treat renal osteodystrophy.10
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


