Chemotherapy and Radiation Therapy
Peter C. Adamson and Larry E. Kun
Over the last four decades of the 20th century, the cure rates for childhood cancer increased from less than 30% to almost 80%.1 Remarkably, most of the advances did not occur from the discovery of new drugs; rather, they came from a better understanding of the underlying biology of childhood cancers; from improvements in the supportive care necessary for the delivery of multimodality therapy; and from improvements in our understanding and use of chemotherapy, radiotherapy, and surgery.  An understanding of the principles of therapy and management of the inherent toxicities of cancer therapy are therefore important for pediatricians today.
An understanding of the principles of therapy and management of the inherent toxicities of cancer therapy are therefore important for pediatricians today.
PRINCIPLES OF CANCER CHEMOTHERAPY
There are three fundamental principles of cancer chemotherapy that are the cornerstone of successful treatment: (1) combination chemotherapy, (2) dose intensity, and (3) adjuvant and neoadjuvant chemotherapy.
 COMBINATION THERAPY
COMBINATION THERAPY
Systemic treatment with chemotherapy works best when a combination of several agents is used. The principle of combining agents with different mechanisms of action, especially those that are synergistic on a mechanistic basis, was defined early in the development of successful childhood leukemia therapy, the combination of methotrexate (MTX) and 6-mercaptopurine (6MP) a cornerstone of ALL therapy today.
 DOSE INTENSITY
DOSE INTENSITY
Most anticancer drugs have a steep dose-response curve, and small increments in dose can significantly influence a drug’s therapeutic efficacy. For many pediatric cancers, administration of each chemotherapy agent at the maximum dose intensity, defined as the amount of drug administered per unit of time (eg, mg/m2 per week), correlates with an improved outcome.5 With a better understanding of caring for the immunocompromised child,  our ability to deliver chemotherapy at maximal dose intensity has improved significantly.
our ability to deliver chemotherapy at maximal dose intensity has improved significantly.
 ADJUVANT THERAPY
ADJUVANT THERAPY
Chemotherapy is most successful when administered in the adjuvant setting—that is, when there is no evidence of residual disease following local therapy with surgery or radiation therapy to the primary tumor.6 In the setting of localized tumors, adjuvant chemotherapy improves outcome by controlling systemic microscopic disease. Neoadjuvant therapy refers to the initiation of chemotherapy prior to delivering definitive local therapy and continuing that chemotherapy after local treatment. Neoadjuvant therapy can facilitate local tumor control by decreasing primary tumor volume and by allowing the physician to assess the tumor’s sensitivity to chemotherapy. 
PRINCIPLES OF CANCER RADIOTHERAPY
Ionizing radiations are physical agents that may result in cellular damage through complex molecular interactions. Like surgery, radiation therapy is a local modality used primarily to provide local or local-regional tumor control. Like chemotherapy, radiation therapy biologically affects tumor cells to a greater extent than normal cells. The aim of radiation therapy (RT) is to optimize the physical delivery of radiation beams, concentrating radiation dose in targeted regions  while minimizing “unintended dose” to surrounding normal tissues.
while minimizing “unintended dose” to surrounding normal tissues.
In pediatrics, optimal radiation delivery must also limit the potential for radiation carcinogenesis.8 The time-dose relationship of fractionated irradiation (treatment administered in daily fractions to accumulate a known tumoricidal dose over several weeks) provides a biological advantage of tumor lethality relative to normal tissue injury.9,10 Radiation therapy may be used as primary therapy but in children is more often used in combination with surgery or chemotherapy. In the unique setting of bone marrow transplantation, total body irradiation (TBI) acts as a systemic agent in conjunction with chemotherapy as part of the conditioning regimen. 
In practice, pediatric radiation therapy begins with the initial patient evaluation, using clinical and imaging data to identify the tumor extent. When surgery or chemotherapy precedes radiation therapy, the targeted volume often includes the initial tumor extent for a proportion of the dose and the postoperative or postchemotherapy residual for a somewhat higher dose. The therapy planning process includes simulation, most often CT to establish patient positioning and immobilization molds.11 The radiation oncologist uses all relevant imaging to outline the appropriate target. Critical normal tissue structures are also designated. A combination of physicists and dosimetrists then plan a series of beam trajectories that overlap in the targeted region; the result is a treatment plan that provides a physical advantage in concentrating the higher dose regions within the tumor and limiting doses received by critical normal structures. Three-dimensional conformal RT (3D-CRT) closely conforms a homogeneous dose to the target; intensity modulated RT (IMRT) adds the possibility of multiple beam subsets with differing dose concentrations in a way that better spares normal structures.12,13-16
Treatment delivery approaches include external beam irradiation or brachytherapy (implanting radioactive sources). Recent technology uses a cyclotron to produce high-energy protons, heavier charged particles compared to photons, which provide a more advantageous dose distribution capable of better concentrating the intended dose within the targeted region.17,18
MECHANISM OF ACTION OF ANTICANCER DRUGS
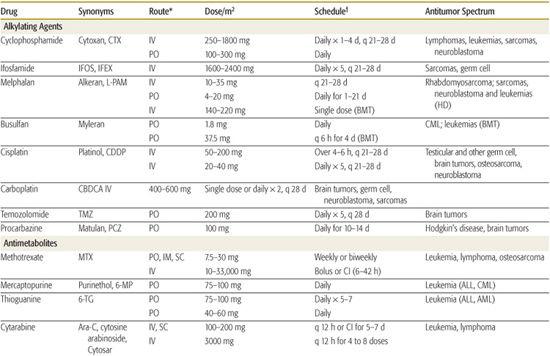
Most commonly used anticancer drugs can be grouped into four broad categories based on their mechanism of action: alkylating agents, antimetabolites, topoisomerase inhibitors, and tubulin-binding agents.5 Within each category there are subgroups of chemically related agents (analogs) that share many characteristics but also have pharmacological properties that make them distinct from their chemically similar relatives.
The majority of cytotoxic drugs produce their effect by interfering with the synthesis or function of DNA and RNA. The largest class of anticancer drugs, the alkylating agents (eg, cyclophosphamide, ifosfamide, melphalan), are chemically reactive compounds that damage DNA by forming covalent bonds to cross-linking nucleobases. These reactions are nonspecific  but it is the reaction with DNA that primarily underlies the cytotoxic effect.
but it is the reaction with DNA that primarily underlies the cytotoxic effect.
The antimetabolites (eg, 6-mercaptopurine, methotrexate, cytarabine) are close structural analogs of the nucleoside precursors of DNA and RNA or analogs of cofactors involved in the synthesis of these building blocks; they either deplete precursors or are incorporated into DNA or RNA as fraudulent substrates. Antimetabolites are cell-cycle specific and thus produce greater cytotoxicity when administered on a continuous or protracted schedule.
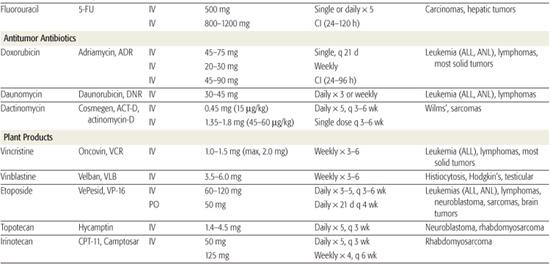
Topoisomerases orchestrate the topology of DNA, creating and relegating single-stranded or double-stranded breaks in the DNA  . A number of anticancer drugs can inhibit these critical enzymes, including the antitumor antibiotics (doxorubicin, actinomycin-D), the epipodophyllotoxins (etoposide and teniposide), and the camptothecins (topotecan, irinotecan).
. A number of anticancer drugs can inhibit these critical enzymes, including the antitumor antibiotics (doxorubicin, actinomycin-D), the epipodophyllotoxins (etoposide and teniposide), and the camptothecins (topotecan, irinotecan).
Tubulin is another major target of cancer chemotherapy. An important function of microtubules is the formation of the mitotic spindle. The vinca alkaloids (vincristine, vinblastine) that inhibit tubulin by blocking microtubule polymerization have widespread efficacy and are used in the treatment of almost all forms of childhood cancer (Table 445-1). 
MECHANISM OF ACTION OF RADIOTHERAPY
Radiation cytolethality is a consequence of direct or indirect irreparable damage to DNA. Tumor histiotypes and normal tissues demonstrate differing degrees of inherent radiosensitivity, rates of repair of sublethal damage and milieu (cellularity, vascularity, reperfusion and oxygenation, necrosis).16,39,40 Such variability explains the differences in radioresponsiveness of tumors and normal tissues and of different tumor types.16,41
Such variability explains the differences in radioresponsiveness of tumors and normal tissues and of different tumor types.16,41
Ionizing radiations damage DNA directly or through free radicals. Resultant single-strand breaks are often repaired, but double-strand breaks more often undergo incomplete or mismatch repair.42 Chromosomal changes result in loss of reproductive capability or programmed cell death (apoptosis).43-45
Chromosomal changes result in loss of reproductive capability or programmed cell death (apoptosis).43-45 Chromosomal instability syndromes, most notably ataxiatelangiectasia, are associated with diminished molecular detection of DNA damage, absent cell-cycle checkpoints, and increased cell death and mutation.46
Chromosomal instability syndromes, most notably ataxiatelangiectasia, are associated with diminished molecular detection of DNA damage, absent cell-cycle checkpoints, and increased cell death and mutation.46
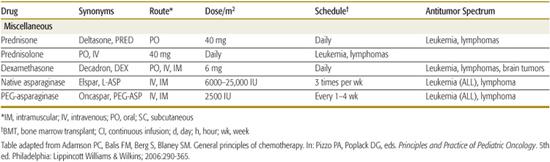
Table 445-1. Commonly Used Drugs for Childhood Cancer
A fractionated radiation course produces a differential response in normal versus tumor cells based upon differences in the amount of cell kill per radiation dose and the repair rates of sublethal damage.48-52 Lower doses per fraction, or lower rates of dose delivery, result in higher cell survival.53 For many growing tissues in children, there is an advantage in reducing the dose per fraction, often enhancing the differential effect and further limiting the late effects that define dose limitations in radiation therapy. During a protracted course of fractioned RT, tumor cell kill results in reoxygenation which is important, as hypoxic cells are inherently less radiosensitive.57Radiation sensitizers by definition potentiate radiation effects and most target hypoxic cells to reduce the impact of relatively radioresistant but viable hypoxic tumor cells.58-61 Most cytotoxic chemotherapy agents have some degree of radiosensitization, clinically most apparent with actinomycin-D, doxorubicin, or cis-platinum.62-64
PREVENTION AND TREATMENT OF ACUTE CHEMOTHERAPY-INDUCED TOXICITY
A primary role of pediatric oncologists is to manage the adverse effects associated with cancer therapy. Unfortunately, more than 80% of children treated for high-risk malignancies experience serious, life-threatening or fatal toxicity during their course of therapy.71 Myelosuppression, nausea and vomiting, and mucositis are associated with a breadth of chemotherapeutic drugs, whereas certain other toxicities  are associated only with distinct drugs or classes of drugs.
are associated only with distinct drugs or classes of drugs.
 MYELOSUPPRESSION
MYELOSUPPRESSION
The ability to routinely support cancer patients with platelet transfusion was a significant advance in supportive care made during the 1960s.72,73 The advent of colony stimulating factors (G-CSF, GM-CSF) in the 1980s decreased the incidence and duration of hospitalization for many patients.74,75 Despite advances in supportive care, hospitalization for febrile neutropenia (defined as fever greater than 38.5°C and an absolute neutrophil count [ANC] of less than 500/mm3 or 1000/mm3 and falling) remains a frequent complication of therapy, necessitating administration of broad-spectrum antibiotics most often given in the inpatient setting.78
Despite advances in supportive care, hospitalization for febrile neutropenia (defined as fever greater than 38.5°C and an absolute neutrophil count [ANC] of less than 500/mm3 or 1000/mm3 and falling) remains a frequent complication of therapy, necessitating administration of broad-spectrum antibiotics most often given in the inpatient setting.78
 NAUSEA AND VOMITING
NAUSEA AND VOMITING
The pediatric oncologist has a significant armamentarium for preventing and treating therapy-induced nausea and vomiting. The cornerstone of therapy includes administration of 5-hydroxytryptamine-3 (5-HT3) receptor antagonists. 
Another very effective antiemetic that has been recently approved for commercial use in the United States is the substance P inhibitor, aprepitant. More traditional emetogenic regimens include a combination of antiemetics is usually employed including the benzodiaze-pines (lorazepam), the H1 histamine receptor antagonists (diphenhydramine, hydroxyzine), and the corticosteroid dexamethasone.80 The dopamine antagonists (droperidol, promethazine, metoclopramide) are used less frequently today because of their extrapyramidal and sedative side effects. 
 MUCOSITIS
MUCOSITIS
Mucositis is common not only to several chemo-therapeutic drugs, but also to radiation therapy that involves the orogastrointestinal mucosa. Chemotherapeutic drugs that cause mucositis primarily include the antimetabolites methotrexate and 5-fluorouracil (5-FU), the anthracyclines doxorubicin and daunomycin, and the camp-tothecin irinotecan (which primarily produces diarrhea). Treatment is mainly supportive.82 The clinician should always be cognizant of infectious causes, primarily herpes simplex, which can present with painful oral lesions and can masquerade as therapy-induced mucositis. 
 DRUG-SPECIFIC TOXICITIES
DRUG-SPECIFIC TOXICITIES
A number of agents induce specific toxicities that can be minimized with appropriate preventive care. Hemorrhagic cystitis is a toxicity that is unique to the oxazaphosphorines cyclophosphamide and ifosfamide. This toxic effect appears to be caused by the activated metabolites and by the biologically active by-products (acrolein) excreted in the urine.84,85 The incidence and severity of chemical cystitis can be lessened by aggressive hydration and by the concurrent administration of mesna (2-mercaptoethane sulfonate).86
The anthracyclines doxorubicin and daunomycin can cause acute and chronic cardiac toxicity. The incidence of clinically apparent congestive heart failure starts increasing after cumulative doses exceed 450 mg/m2 for doxorubicin and 700 mg/m2 for daunomycin.87,88 Children appear to be at higher risk for cardiac toxicity, and those younger than 5 years are at higher risk than older children.89-91 Children receiving anthracycline therapy should have their cardiac function closely monitored. 
Although cisplatin is associated with only mild myelosuppression, it produces significant and potentially irreversible nephrotoxicity, ototoxicity, and neurotoxicity. Nephrotoxicity is manifested as azotemia and electrolyte disturbances, especially hypomagnesemia.94-96 Renal damage from cisplatin is cumulative. Although pretreatment hydration, diuresis, chloruresis, and less-toxic dose schedules have reduced the incidence and severity of cisplatin-induced nephrotoxicity, moderate and permanent reductions in the glomerular filtration rate of patients receiving cisplatin still occur.97-99
PREVENTION AND TREATMENT OF RADIOTHERAPY-INDUCED TOXICITY
While the biological effect of radiation exposure is immediate (measured in microseconds during exposure), the clinical effects may be apparent at widely varying times during radiation therapy (RT) (immediate effects), soon after (subacute effects, typically within 1 to 6 months post-RT), or late-post-RT (late effects, typically beyond 1 to 2 years after therapy). Most acute reactions can be handled symptomatically and supportively, resolve during or within a few weeks after RT, and are associated with late effects only if pronounced. Many acute reactions can be limited by the radiation technique, intensive local care, or sometimes discontinuing concurrent drug therapies that may enhance radiation responses. 
Subacute reactions are largely visceral changes that reflect later manifestations of changes in tissue proliferation or the impact of specific cell types (eg, type 2 pneumocytes, renal tubular cells, myelin-producing astrocytes) that produce clinically apparent changes weeks or months after radiation therapy.100-105 Such effects in part reflect cytokine-related chronic inflammatory changes. Depending on the volume of the organ subtended, the radiation dose, and the individual sensitivity, subacute postirradiation changes (pneumonitis, early nephropathy, hepatopathy, or central nervous system white matter effects) may be apparent only by imaging or functional tests, or may be symptomatic or even life-threatening. 
The late effects of radiation therapy define dose limits and technical approaches to avoid serious consequences. Late effects include changes in organ function and in growth and development (physical and neuropsychological) and secondary cancer production.107,108 Normal tissue tolerances relate to specific tissue types; the volume of the organ or tissue irradiated; and the radiation dose to that structure. 
The underlying pathophysiology relates to direct effects on parenchymal cells and indirect effects usually mediated through changes in small arterial vessels.100 Late effects include focal brain necrosis or white matter degeneration in the CNS, alterations in epiphyseal physiology resulting in stunted bone growth, reduction in pulmonary function due to fibrosis and alterations in the capillary-alveolar membranes, diminished or ablated hormonal production, cardiovascular stenosis and valvular dysfunction, limited capacities and sometimes recurrent hemorrhage or ulceration in the urinary bladder or colon/rectum, and cataract production, among other specific post-RT “syndromes.”102,109-111 Symptomatic management is organ- or effect-dependent. 
Postirradiation carcinogenesis is a risk attendant even to low doses of radiation exposure in children. Most second cancers are late epithelial cancers, typically arising more than 15 years after irradiation.115,116 The overall risk of second cancers ranges from 25% to 30% at 20+ years in children with genetic predisposition (eg, bilateral retinoblastoma) to nearly 20% for breast cancer in girls exposed during the pubertal years to 4% to 7% overall in the general pediatric population.111,117,118
REFERENCES
See references on DVD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


