Treatment of gynecologic cancer typically requires a multidisciplinary and multitreatment approach involving a combination of surgery, chemotherapy, and radiation therapy. When more than one modality is used, they may be delivered sequentially or at the same time, as with chemoradiation or intraoperative radiation therapy. The sequence of treatment is characterized as “primary,” referring to initial treatment; “adjuvant,” referring to secondary treatment for micrometastatic disease after surgical management; “neoadjuvant,” referring to induction chemotherapy, radiation therapy, or both administered before definitive therapy; and “salvage,” referring to treatment at time of recurrence.
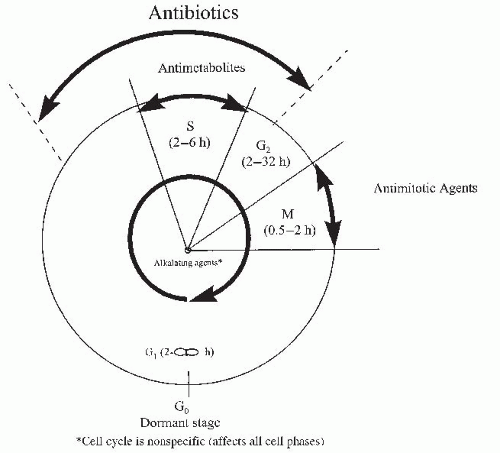
Tumor cells grow as a result of deregulation between proliferation and suppression. Our understanding of cancer cell kinetics and the classical cell cycle (Fig. 50-1) has led to the development of several chemotherapy drugs. There are both cell cycle-specific chemotherapeutic agents and cell cycle-nonspecific chemotherapeutic agents.
Cell cycle-specific agents depend on the proliferative capacity of the cell and the phase of the cell cycle for their action. They are effective against tumors with relatively long S phases and rapid proliferation rates.
Cell cycle-nonspecific drugs kill cells in all phases of the cell cycle and their effectiveness is not dependent on proliferative capacity. Radiation therapy is not cell cycle dependent.
Chemotherapeutic agents commonly used for the treatment of gynecologic cancer may be grouped into the following categories (Table 50-1):
Alkylating agents are cell cycle-nonspecific. They contain an alkyl group that forms a covalent bond with the DNA helix, preventing DNA duplication. They
also function by attaching to free guanine bases of DNA thereby prohibiting their action as templates for new DNA formation.
Antimetabolites are similar in chemical structure to compounds required by normal and tumor cells for cell division. These antimetabolites may be incorporated into new nuclear material or combined with enzymes to inhibit cell division.
Plant alkaloids are derived from various plants and trees, including the periwinkle plant (Vinca rosea), the mayapple (Podophyllum peltatum), and the Pacific yew (Taxus brevifolia). They bind to tubules, blocking microtubule formation, and interfering with spindle formation. This leads to the arrest of metaphase and inhibits mitosis.
Antitumor antibiotics have many different modes of action, including increasing cell membrane permeability, inhibiting DNA and RNA syntheses, and blocking DNA replication.
Biologics target known mutations to oncogenic signal transduction pathways specific to cancer cells. As cancer biology is further elucidated, increasing numbers of biologics are being discovered and tested.
Miscellaneous agents have different modes of action than those previously mentioned.
TABLE 50-1 Chemotherapeutic Agents Frequently Used in Gynecologic Cancer and Their Most Common Toxicities | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Hematologic toxicity and myelosuppression is a dangerous effect of chemotherapy that varies in severity depending on the drug administered. A nadir in white cell, red cell, or platelet count is usually observed 7 to 14 days after drug administration. Most agents are readministered every 3 to 4 weeks if the patient has recovered from pancytopenia.
Neutropenia is defined as an absolute neutrophil count (ANC) less than 500/mL. Recombinant human granulocyte colony-stimulating factor (G-CSF) (filgrastim, Neupogen) or pegylated filgrastim (Neulasta) is administered to at-risk patients or the cycle after neutropenia is diagnosed as prophylaxis against this reaction in subsequent cycles. Use of G-CSF is contraindicated during the actual administration of chemotherapy or during a neutropenic fever.
Neutropenic fever is a medical emergency, as these patients can quickly become septic and decompensate. Common causes of infection include enteric Gramnegative bacteria, Gram-positive bacteria (Staphylococcus epidermidis, Staphylococcus aureus, and diphtheroids), viruses (herpes simplex and herpes zoster), and
fungi (Candida and Aspergillus species), although often an offending agent is not identified. Infections are generally due to reduced integrity of the mucous membranes and skin during chemotherapy. Once fever (temperature greater than 38°C) is noted, broad-spectrum antibiotics with antipseudomonal coverage should be initiated immediately.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree