Midline Vertical Incision
Abdominal entry is the first step for many gynecologic surgeries. Either vertical or transverse incisions may be used, and each offers particular advantages. Vertical incisions may be midline or paramedian, but of the two, the midline is chosen more often. This incision offers quick entry, minimal blood loss, superior access to the upper abdomen, generous operating room, and the flexibility for easy wound extension if greater space or access is needed. No important neurovascular structures traverse this incision. Thus, it may be favored for patients using anticoagulation agents. Despite advantages, midline incisions are more frequently associated with greater postoperative pain, poorer cosmetic results, and increased risks of wound dehiscence or incisional hernia compared with low transverse incisions. Last, for those with prior laparotomy, the incision type is typically repeated for subsequent surgeries.
For abdominal entry, patients are informed of wound infection or dehiscence risks. Additionally, the chance of bowel or bladder injury is present with any abdominal entry, especially when extensive adhesions are encountered.
Laparotomy per se does not require antibiotic prophylaxis or bowel preparation. These are dictated by the planned procedure. Prevention for venous thromboembolism is warranted, and options are described in Chapter 39.
After administration of adequate regional or general anesthesia, the patient is positioned supine. If needed, hair in the path of the planned incision is clipped; a Foley catheter is placed; and abdominal preparation is completed.
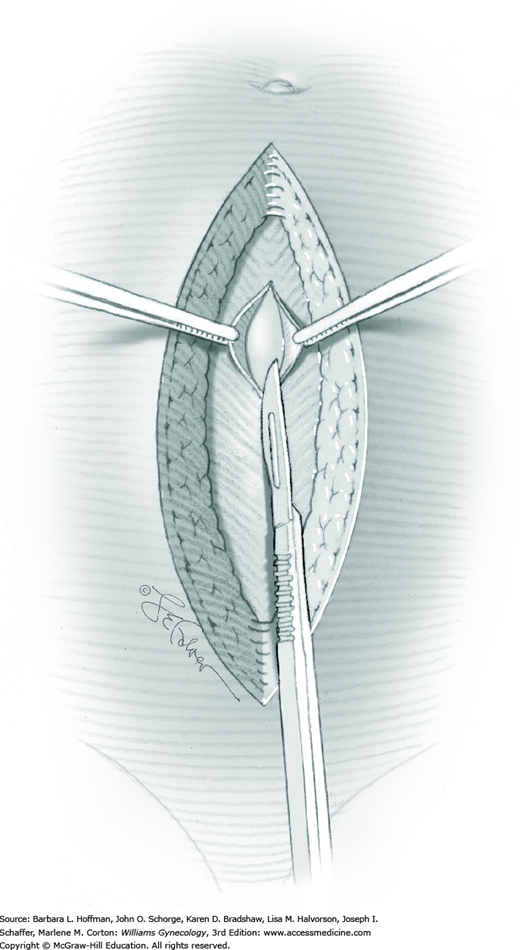
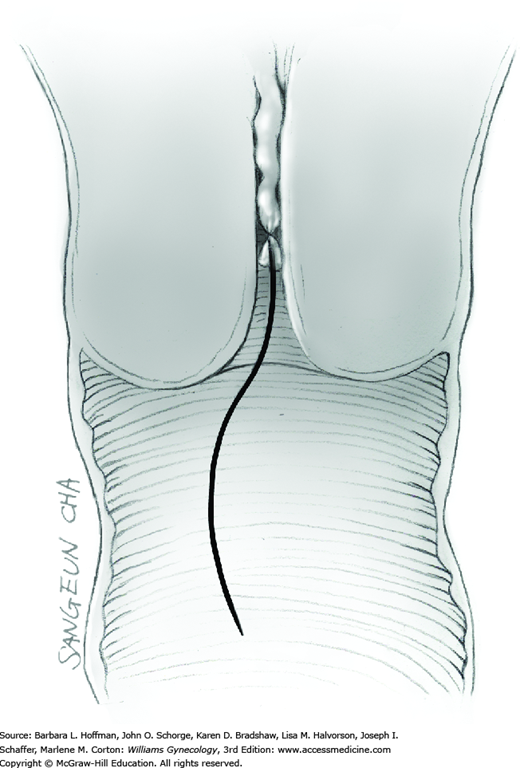
The skin is incised vertically in the midline beginning 2 to 3 cm above the symphysis pubis and extending cephalad to within 2 cm of the umbilicus. If less space is required, this incision may be shortened. For greater space or access, the incision may arch around the umbilicus and then continue cephalad in the upper abdominal midline. This extension passes to the left of the umbilicus to avert transection of the ligamentum teres. This remnant of the umbilical vein courses in the free border of the falciform ligament. The umbilicus itself contains attenuated fascia. Thus, the periumbilical incision should arch laterally enough to provide quality fascia on either side of the incision to allow an ultimately secure closure.
The subcutaneous layers of Camper and Scarpa are then incised either sharply with long even strokes or with electrosurgical blade to reach the linea alba fascia. Ideally, the number of blade strokes is minimized to avoid hatch marking the tissue, which increases tissue damage and wound infection risks.
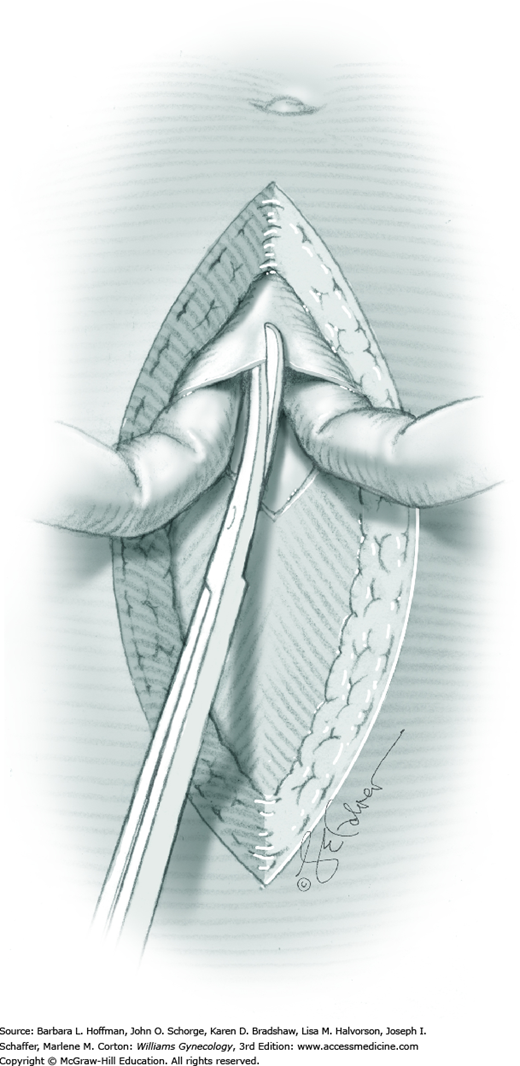
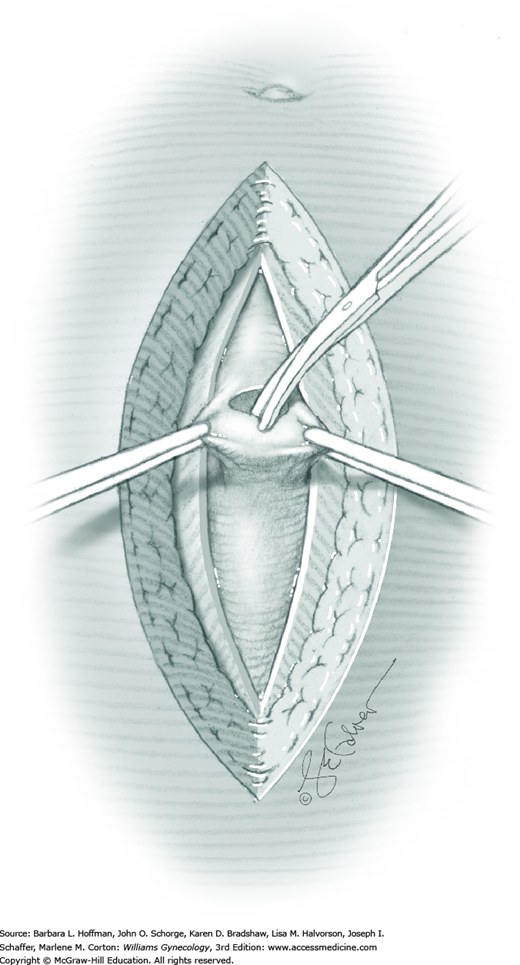
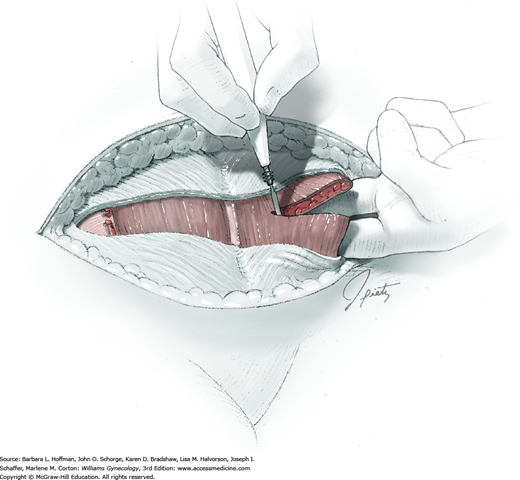
Tendinous fibers from the anterior abdominal wall aponeuroses merge in the midline of the abdomen to form the linea alba. This fascia layer is sharply entered near the midpoint of the incision (Fig. 43-1.1). This incision is extended first cephalad and then caudally to mirror the length of the skin incision. To minimize injury to viscera during this extension, the linea alba is elevated by index fingers of the surgeon and assistant or by the open tips of a pean clamp (Fig. 43-1.2).
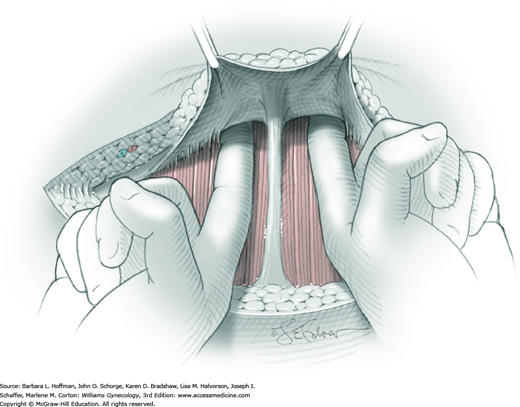
Once the fascia is incised, the right and left rectus abdominis muscle bellies are bluntly separated laterally. Inferiorly, sharp incision may be required to complete this. To avert injury to organs below, the rectus muscles are elevated during this division. Each half of the pyramidalis muscle lies atop its respective rectus belly, and this muscle is similarly divided sharply at the midline.
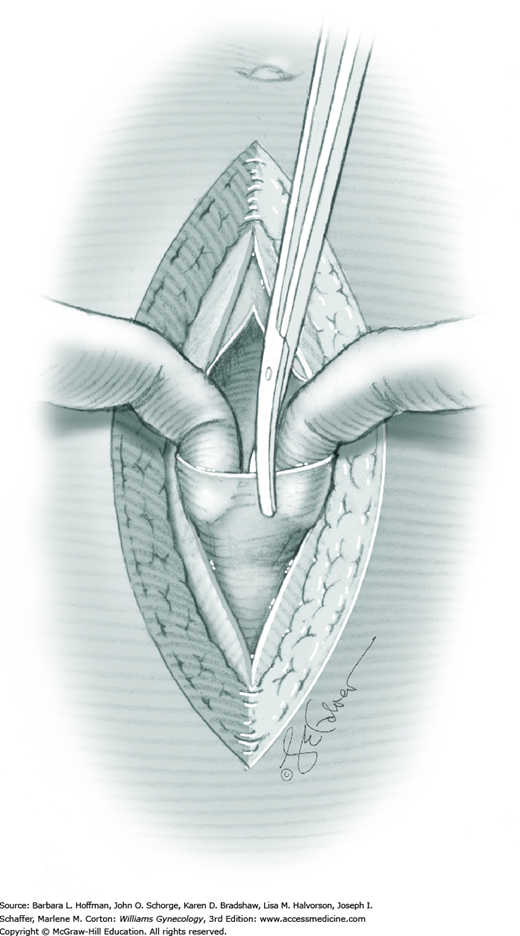
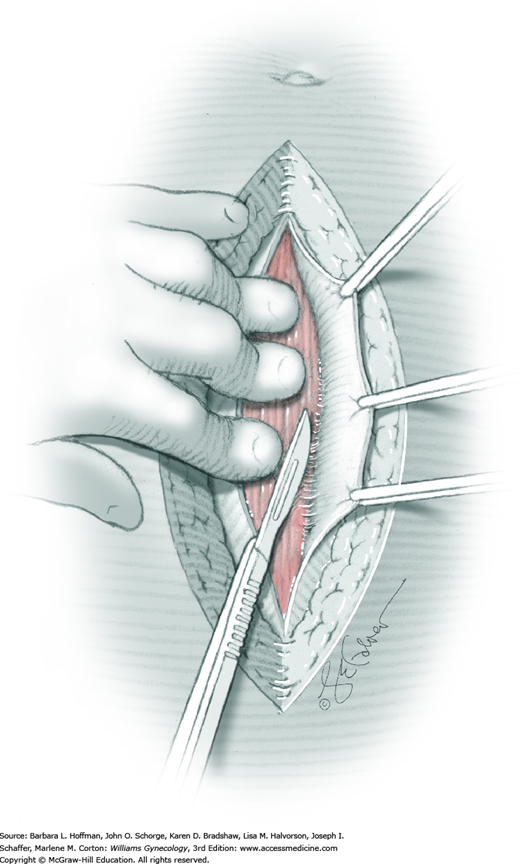
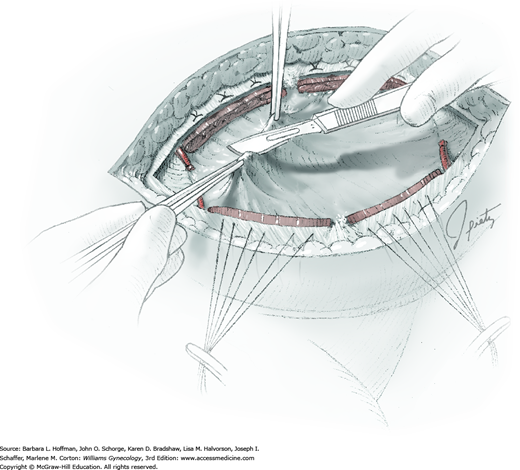
For entry, the peritoneum is identified between the rectus abdominis muscle bellies. It is grasped with two hemostats in the upper portion of the incision to avoid cystotomy. The interposed peritoneum should be palpated or visually examined to exclude intervening viscera. Only then is it sharply cut (Fig. 43-1.3). Next, an index finger sweeps directly beneath it to identify adhered bowel or omentum. If free, the peritoneum is elevated by fingers of the surgeon and assistant to protect viscera beneath. As the incision is extended cephalad above the arcuate line, the transverse fibers of the posterior rectus sheath are seen and are cut along with the peritoneum. As the incision is extended caudally, the transversalis fascia is found superficial to the peritoneum. To prevent bladder dome injury, this thin fascia layer is elevated by insinuating fingers beneath it and is cut, with the incision extending caudally. Next, the peritoneum beneath the transversalis fascia is similarly incised (Fig. 43-1.4). The bladder dome is identified by increasing tissue vascularity and thickness. Of note, the urachus, which is the remnant of the allantois, may be seen as a white cord extending from the bladder dome toward the umbilicus in the midline.
During abdominal entry, prior surgery may blur clear tissue planes. For example, the true midline may be deviated laterally, and after fascial incision, only rectus fibers may be seen. To find the midline after incising the fascia, fibers of the pyramidalis muscles can be followed as they angle toward the midline. Also, visually recreating a line between the symphysis and umbilicus can aid orientation. To reach the midline, the fascia closest to the presumed midline is grasped both cephalad and caudad along its length with Kocher clamps to create upward tension (Fig. 43-1.5). Simultaneously, downward manual pressure atop the ipsilateral rectus belly accentuates fibers between the fascia and underlying rectus belly. These fibers are then cut to permit lateral dissection of the fascia away from the rectus belly. This is continued laterally until the midline is identified. If the midline is not identified after some dissection on one side, the same steps can be repeated on opposite side.
Also with prior surgery, planes between the fascia, peritoneum, and viscera may be poorly defined. In these cases, a gradual layered entry is required to avoid organ injury. One technique uses Metzenbaum scissors. Scissor tips are insinuated between tissue layers such that the tips are seen each time prior to cutting. This minimizes the risk that the thicker bowel or bladder wall will be cut. If adhesions are found, they are divided. Dissection is maintained close to the fascial or peritoneal edge to minimize visceral injury.
After abdominal entry, a self-retaining retractor is commonly placed to retract the bowel, omentum, and abdominal wall muscles. Moist laparotomy sponges are placed around the bulk of bowel and gently directed cephalad. Adhesiolysis may be required to adequately free intestines for this repositioning. Upper blades of the retractor assist in holding these loops up and away from the pelvis and operating field. The shortest blades possible are preferred for lateral retraction. This reduces the risk of femoral or genitofemoral nerve compression by a blade resting atop the psoas major muscle. Once pelvic organs are adequately exposed, the planned abdominal surgery can proceed.
Closure of the visceral or parietal peritoneum is not required and is individualized (Chap. 40). Starting from each end of the incision, the fascia is closed to its midpoint using a continuous running suture with a 0-gauge delayed-absorbable suture. These sutures are then tied together. If the subcutaneous layer measures less than 2 cm, then no closure is typically necessary. For deeper wounds, interrupted stitches of 2-0 to 4-0 gauge absorbable or delayed-absorbable suture are used to close this layer. The skin is closed with a subcuticular stitch using 4-0 gauge delayed-absorbable suture, staples, or other suitable method (Chap. 40).
For most gynecologic surgeries, recovery from the abdominal incision constitutes the greatest portion of postsurgical healing. Midline incisions lead to significant pain during ambulation, coughing, and deep breathing. As a result, women undergoing laparotomy are at greater risk of postoperative thrombotic and pulmonary complications. Prevention of these is warranted and described in Chapter 42. In addition, return of normal bowel function is commonly slowed, and signs of ileus should be monitored. Hospitalization typically varies from 1 to 3 days, and return of normal bowel function usually dictates this course. Postoperative activity in general can be individualized, although vigorous abdominal exercise is delayed for 6 weeks to allow for fascial healing. Driving can be resumed when pain does not limit the ability to brake quickly and when narcotic medications are not in use. Return to work is variable, although 6 weeks is commonly cited.
Pfannenstiel Incision
The Pfannenstiel, Cherney, and Maylard incisions are transverse abdominal incisions used for gynecologic procedures. Of these, the Pfannenstiel incision is the most commonly used incision for laparotomy in the United States. Because the transverse incision follows Langer lines of skin tension, excellent cosmetic results can be achieved. Additionally, decreased rates of postoperative pain, fascial wound dehiscence, and incisional hernia are noted. Use of the Pfannenstiel incision, however, is often discouraged for cases in which greater operating space or upper abdominal access is anticipated. Last, because of the layers created by incision of the internal and external oblique aponeuroses, purulent fluid can collect between these. Therefore, cases involving abscess or peritonitis favor use of a midline incision, which divides the fused linea alba.
General risks associated with transverse laparotomy incisions are similar to those for vertical incisions. Transverse incisions, however, carry risk of injury to the iliohypogastric and ilioinguinal nerves. These injuries frequently involve only transient sensory loss but rarely may lead to debilitating, chronic neuropathic pain.
After administration of adequate regional or general anesthesia, the patient is positioned supine. If needed, hair in the path of the planned incision is clipped; a Foley catheter is placed; and abdominal preparation is completed.
Two to 3 cm above the symphysis pubis, an 8- to 10-cm transverse incision is made with its lateral margins arching slightly cephalad. If less space is required, this length can be shortened. The incision is deepened sharply with scalpel or electrosurgical blade until the anterior rectus sheath is reached. The superficial epigastric vessels typically lie several centimeters from the midline and halfway between the skin and fascia. Coagulation of these vessels will limit incisional blood loss.
The anterior rectus sheath is sharply incised transversely in the midline (Fig. 43-2.1). At the level of the incision, the anterior rectus sheath is composed of two visible layers, the aponeuroses from the external oblique muscle and a fused layer containing aponeuroses of the internal oblique and transversus abdominis muscles. Lateral extension of the anterior rectus sheath incision requires cutting each layer individually (Fig. 43-2.2). This permits identification and ideally, avoidance of the iliohypogastric and ilioinguinal nerves as they run between these two fascial layers.
Of note, at the level of the incision, the inferior epigastric vessels typically lie outside the lateral border of the rectus abdominis muscle and beneath the fused aponeuroses of the internal oblique and transversus abdominis muscles. Thus, lengthening the incision farther laterally may cut these vessels. If significant lateral extension is required, these vessels are identified, clamped, and ligated. This prevents bleeding and vessel retraction with later hemorrhage. In addition, risk of iliohypogastric and ilioinguinal nerve injury also increases as the incision is carried lateral to the rectus abdominis muscle borders (Rahn, 2010).
The superior edge of the fascial incision is grasped with a Kocher clamp on either side of the midline. Traction is directed cephalad and slightly outward. In the area superior to the initial incision, the anterior rectus sheath is then bluntly or sharply separated from the underlying rectus abdominis muscle (Fig. 43-2.3). With blunt dissection, upward facing fingers first push cephalad and then roll to direct pressure laterally. The fascia separates easily from the bellies of the rectus muscle, but it may be densely adhered along the midline and require sharp dissection (Fig. 43-2.4). Several small perforating nerves and vessels traverse the space between the anterior rectus sheath and rectus muscle. These vessels are coagulated to avoid laceration and bleeding. Upon completion of this dissection, a semicircular area with a radius of 6 to 8 cm has been created. A similar separation is performed in the area inferior to the initial fascial incision.
The rectus abdominis muscle bellies are then separated laterally from the midline either bluntly or sharply. The pyramidalis muscle, located superficial to the rectus muscle, usually requires sharp division at the midline.
Peritoneal entry is fully described and illustrated in Section 43-1, Steps 3 and 4. To summarize, upon separation of the rectus muscle bellies, the thin, filmy peritoneum is identified, grasped with two hemostats, and sharply incised. The peritoneal incision is then extended superiorly and inferiorly. Once the abdominal cavity has been entered and clear visualization established, the surgeon can proceed with the planned operation.
Closure of the visceral or parietal peritoneum is not required and is individualized. Starting from each end of the incision, the fascia is closed to its midpoint using a continuous running suture line with a 0-gauge delayed-absorbable suture (Fig. 43-2.5). These strands are then tied together. The subcutaneous layer and skin are closed similar to the midline vertical incision (Section 43-1).
The postoperative course for low transverse incisions follows that described for midline incisions.
Cherney Incision
The Cherney incision is a transverse abdominal incision that is similar to the Pfannenstiel incision in its early steps. After the anterior rectus sheath is opened, however, the tendons of the rectus abdominis and pyramidalis muscles are transected 1 to 2 cm above their insertion into the symphysis pubis. These muscles are then lifted cephalad to provide access to the peritoneum. At this level, the inferior epigastric vessels run well lateral to the rectus bellies and typically are spared. However, if additional lateral extension is required, these vessels are ligated and transected.
This incision offers generous operating space and access to the space of Retzius. Thus, it may be a primary choice when these requirements are anticipated. Additionally, Pfannenstiel incisions may be converted to Cherney incisions when an unexpected need for additional space arises.
Preparation and consenting prior to Cherney incision are similar to that for Pfannenstiel incision.
The initial steps mirror that of the Pfannenstiel incision (Steps 1-3). Thus, the skin is incised transversely beginning 2 to 3 cm above the symphysis, the fascia is divided transversely, and the rectus sheath is dissected off the rectus abdominis muscle bellies. After these steps, however, the techniques diverge.
The fascial opening reveals the rectus abdominis and pyramidalis muscles. Cephalad to the symphysis pubis, fingers are insinuated beneath the rectus muscle tendons. This blunt dissection begins laterally and extends toward the midline. During insinuation, fingers exert pressure dorsally and against the bladder to protect it during tendon division. After this, fingers lift up the muscle tendons, which are then transected 1 to 2 cm above the symphysis pubis (Fig. 43-3.1). The muscles are flipped up and cephalad.
The peritoneum is grasped with two hemostats at a level above the bladder dome and is sharply incised. This incision is extended laterally. Once the abdominal cavity has been accessed, the planned surgery can proceed. Importantly, the risk of injury, particularly to the femoral and genitofemoral nerves, is increased when self-retaining retractors are used with this generally wider incision. This is also true for the Maylard incision. Thus, lateral retractor blades should fit just under the edges of the incision and not rest atop the psoas muscle.
During wound closure, the cut ends of the rectus muscle tendons are affixed with interrupted sutures of 0-gauge delayed-absorbable sutures to the undersurface of the inferior fascial edge (Fig. 43-3.2). To avoid osteitis pubis or osteomyelitis, the tendons should not be affixed directly to the symphysis pubis.
Starting from each end of the incision, the fascia is closed to its midpoint using a continuous running suture with a 0-gauge delayed-absorbable suture. These strands are then tied together. The subcutaneous layer and skin are closed as with the midline vertical incision.
The postoperative course for low transverse incisions follows that described for midline incisions.
Maylard Incision
The Maylard incision differs mainly from the Pfannenstiel incision in that the rectus sheath is not dissected away from the rectus abdominis muscle and that the bellies of the rectus abdominis muscle are transected. Transection affords extensive access to the pelvis. However, it is technically more difficult due to its required isolation and ligation of the inferior epigastric vessels. Moreover, the Maylard incision has been used infrequently because of concerns regarding greater postoperative pain, decreased abdominal wall strength, longer operating times, and increased febrile morbidity. Randomized studies, however, have not supported these concerns (Ghanbari, 2009; Mathai, 2013). The Maylard incision should be avoided in those patients in whom the superior epigastric vessels have been interrupted, as this leaves the rectus abdominis muscles with inadequate blood supply. Also, patients with significant peripheral vascular disease may rely on the inferior epigastric vessels for collateral blood supply to their lower extremities (Salom, 2007).
Preparation and consenting prior to Maylard incision are similar to that for Pfannenstiel incision.
The initial steps mirror that of the Pfannenstiel incision (Steps 1 and 2). Thus, the skin is incised transversely beginning 2 to 3 cm above the symphysis, and the fascia is divided transversely. After these steps, the techniques diverge, and in contrast to the Pfannenstiel incision, the anterior rectus sheath is not dissected away from the underlying rectus muscle.
The inferior epigastric vessels lie posterolateral to the rectus abdominis muscle bellies. Bilaterally, these vessels are identified, ligated, and transected. This step avoids their laceration and hemorrhage when the rectus abdominis muscle is transected.
With fingers, the rectus abdominis muscle is bluntly dissected away from the underlying transversalis fascia and peritoneum. These latter are the encountered layers because the posterior rectus sheath ends at the arcuate line and is absent caudal to this line. The surgeon’s fingers are slid behind the rectus muscle bellies, and this muscle is then transected using an electrosurgical blade (Fig. 43-4.1).
With two hemostats, the peritoneum is grasped, and it is sharply incised above the level of the bladder dome. This incision is then extended laterally (Fig. 43-4.2). After access is obtained to the abdominal cavity, planned surgery can proceed. As with Cherney incisions, careful self-retaining retractor placement is necessary to lessen the risk of femoral or genitofemoral nerve injury.
At incision closure, the fascia is closed with a running stitch using 0-gauge delayed-absorbable suture. Closing the fascia adequately reapproximates the transected muscle fibers, and therefore the divided muscle bellies are not directly sutured together. Starting from each end of the incision, the fascia is closed to its midpoint using a continuous running suture line with a 0-gauge delayed-absorbable suture. These strands are then tied together. The subcutaneous layer and skin are closed as with the midline vertical incision.
The postoperative course for low transverse incisions follows that described for midline incisions.
Ovarian Cystectomy
Ovarian cyst excision is typically prompted by patient symptoms or by ovarian qualities that suggest a lower concern for ovarian malignancy (Chap. 9). Removal of the cyst alone can offer those with ovarian pathology an opportunity to preserve hormonal function and reproductive capacity. Accordingly, ovarian cystectomy goals include gentle tissue handling to limit postoperative adhesions and reconstruction of normal ovarian anatomy to aid the later transfer of ova to the fallopian tube.
In some women, a cystectomy may be performed laparoscopically rather than with laparotomy. Several studies support the safe and effective use of laparoscopy for this purpose (Chap. 9). That said, there are settings in which its role is limited. In general, if a cyst is large, adhesive disease limits access and mobility, or the risk of malignancy is greater, then laparotomy is preferred.
In addition to general surgical risks of laparotomy, the major risk of cystectomy is extensive bleeding from or injury to the ovary that, in turn, necessitates removal of the entire ovary. Also, a variable degree of ovarian reserve may be lost with ovarian cystectomy. If ovarian cancer is suspected prior to surgery, patients should be educated regarding the possibility of surgical staging, including the need for hysterectomy and removal of both ovaries (Chap. 35).
Many patients undergoing cystectomy for ovarian pathology have associated pain. Although in most cases cystectomy will be curative, in other instances, pain may persist despite cyst excision. This is especially true in those with coexistent endometriosis. Thus, patients are counseled that cystectomy may not relieve chronic pain in all cases.
Bowel preparation and antibiotics are typically not required preoperatively. If hysterectomy is required during ovarian staging, antibiotics may be given intraoperatively. Laparotomy dictates venous thromboembolism prophylaxis, and options are found in Table 39-8.
Because of the potential for cancer staging in the upper abdomen if malignancy is found, general anesthesia is typically indicated for this inpatient procedure. The patient is supine. After anesthesia induction, hair in the planned incision path is clipped if needed; a Foley catheter is inserted; and abdominal preparation is completed. Because hysterectomy may be needed if malignancy is found, the vagina is also surgically prepared.
Most ovarian cysts can be removed through a Pfannenstiel incision. Extremely large cysts or those with a greater concern for malignancy usually require a vertical incision. This latter incision provides generous operating space and adequate upper abdomen access for cancer staging.
As described in Chapter 35, cell washings from the pelvis and upper abdomen are collected prior to ovarian manipulation and are saved if a cancer is found. The upper abdomen and pelvis are explored, and excrescences or suspicious areas are sampled and sent for intraoperative frozen-section analysis.
A self-retaining retractor is placed within the incision, and the bowel and omentum are packed from the operating field. The ovary is brought into view, and moist laparotomy sponges are placed in the cul-de-sac and beneath the ovary. This helps to minimize contamination of the pelvis if the cyst ruptures during excision.
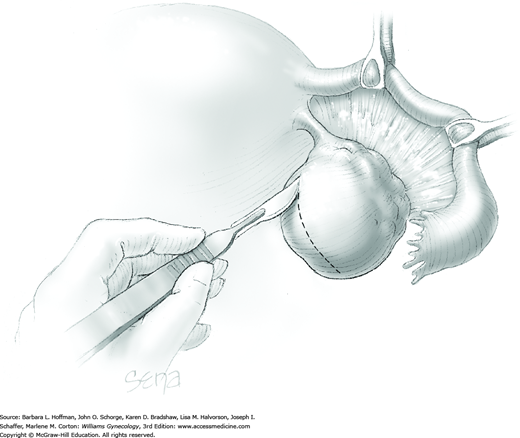
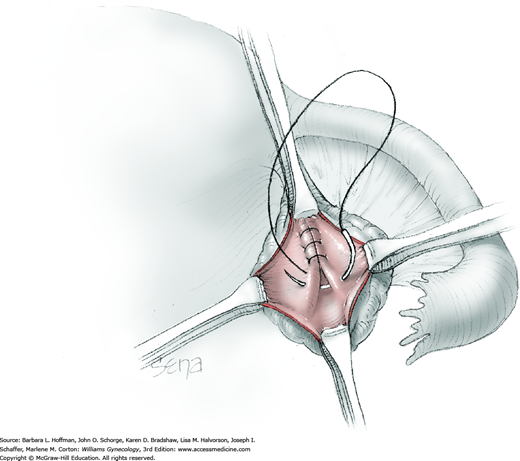
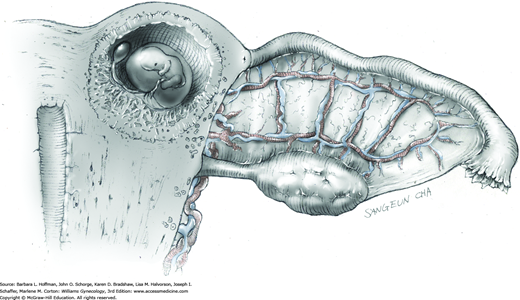
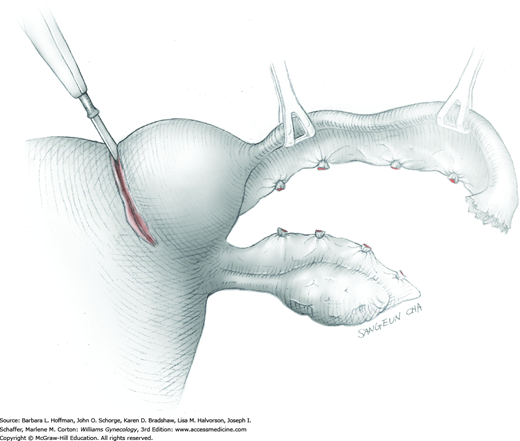
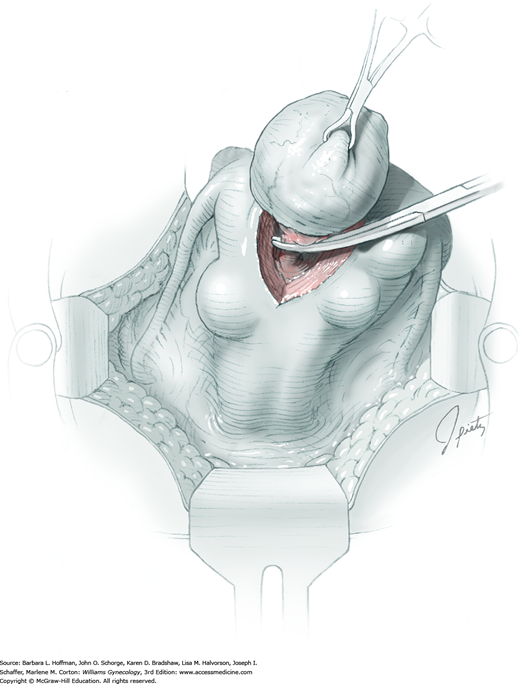
The ovary is held between the surgeon’s thumb and opposing fingers. The ovarian capsule that overlies the dome of the cyst is then cut with either scalpel or electrosurgical needle tip. This incision is ideally placed on the antimesenteric surface of the ovary to minimize dissection into vessels at the ovarian hilum. The incision is ideally deepened to reach the cyst wall without entering and rupturing the cyst (Fig. 43-5.1). Allis clamps are then placed on the incised edges of the ovarian capsule to aid traction and countertraction during dissection.
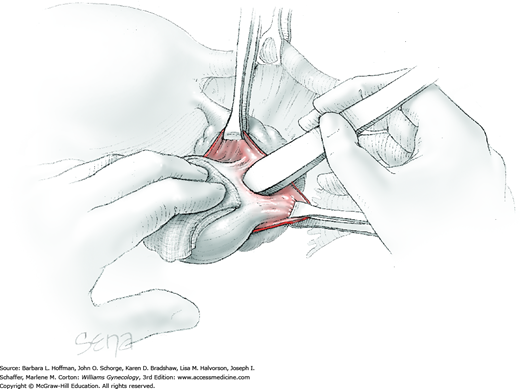
Blunt dissection with fingertip or knife handle or sharp dissection with Metzenbaum scissor tips is used to develop the cleavage plane between the cyst wall and the remaining ovarian stroma (Fig. 43-5.2). If adhesions obliterate the cleavage plane, sharp dissection is preferred. As an assistant gently pulls the Allis clamps in a direction away from the cyst wall, the surgeon places fingers proximate to the advancing cleavage plane and pulls the cyst in the direction opposite the Allis clamps. Such traction and countertraction across the cleavage plane aid dissection. Because the surface of the cyst wall is often smooth and slippery, the surgeon may place an unfolded thin gauze sponge between fingers and the cyst wall to afford a better grip.
As dissection approaches completion, the highly vascular ovarian hilum is reached. If possible, a hemostat or pean clamp is placed across the small remaining tissue bridge between the cyst and normal ovary. The clamp is positioned closer to the ovary to allow space for scissors to cut the tissue pedicle and free the cyst without rupture. The pedicle is suture ligated with a fine absorbable suture. The ovarian bed is then examined, and bleeding points are coagulated or ligated.
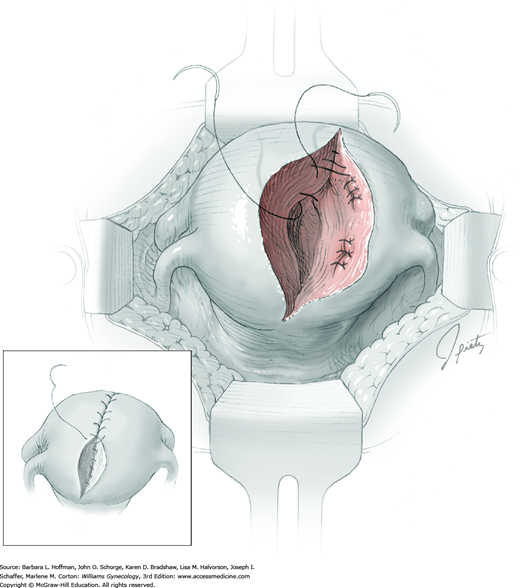
Once the cyst is removed, it may be sent to the pathology department for intraoperative frozen-section analysis. In benign cases, excess capsule can be sharply trimmed from ovaries in which large cysts have stretched and thinned the ovarian surface. This excision is performed to help restore normal ovarian anatomy. But because ovarian follicles are contained within even extremely thinned capsules, this tissue is preserved whenever possible.
The ovarian bed is then closed in layers using 3-0 or 4-0 gauge delayed-absorbable suture. These sutures reapproximate the ovarian tissue that previously surrounded the cyst on both sides (Fig. 43-5.3). With a thinned ovarian cortex, the needle tip should not be driven through the capsule. Exposed suture on the ovarian surface may increase adhesion formation. The ovarian incision is closed with a running subcortical stitch (similar to subcuticular stitch) using 5-0 gauge delayed-absorbable suture.
Laparotomy sponges are removed from the cul-de-sac, and the pelvis is copiously irrigated with an isotonic solution such as lactated Ringer solution. Irrigation assumes an even greater importance with ovarian cyst rupture. For example, spill from a mature cystic teratoma (dermoid), if neglected, may induce a chemical peritonitis. Depending on the surgeon’s preference and the patient’s anatomy, an adhesion barrier may be placed around the ovary (Chap. 11). The remaining packs and retractor are removed, and the abdominal incision is closed.
After surgery, patient care in general follows that described for laparotomy.
Salpingo-oophorectomy
Removal of the ovary and fallopian tube is more commonly performed by laparoscopy. However, laparotomy is typically indicated if the potential for malignancy is great, if the ovary is larger than 8 to 10 cm, or if extensive adhesions are anticipated. With either approach, the essential steps of salpingo-oophorectomy (SO) are: preventive identification of the ipsilateral ureter, infundibulopelvic (IP) ligament ligation, combined ligation of the proximal fallopian tube and uteroovarian ligament, and transection of the intervening mesovarium and mesosalpinx. Indications are varied and include suspicion for ovarian malignancy, ovarian cancer prevention for at-risk women, large symptomatic ovarian cysts in postreproductive females, and for reproductive-aged women, large, symptomatic ovarian cysts that are not suitable for cystectomy.
This surgery is typically performed to remove ovarian pathology that has been evaluated sonographically. If anatomy is unclear, magnetic resonance (MR) imaging may add information. As listed in Chapters 35 and 36, tumor markers are selectively drawn prior to surgery if malignancy is suspected.
In general, serious complications with SO are infrequent but include organ injury, especially to the ureter; hemorrhage; wound infection or dehiscence; and anesthesia complications. Ovarian pathology is the most common indication for SO. Thus, the possibility of cancer staging and a description of its steps are explained. Moreover, malignant cyst rupture and spillage are risks, and patients are informed that this will advance the cancer stage (Chap. 35). Many women undergoing SO for ovarian pathology have associated pain. Although removal of the ovary in most cases will be curative, in other instances, pain may persist despite SO. Last, if performed bilaterally, SO dramatically curtails estrogen production. Thus, a preoperative discussion of consequences, as outlined on page 951, is recommended.
Bowel preparation and antibiotics are typically not required preoperatively. If hysterectomy is required during ovarian staging, antibiotics may be given intraoperatively. Laparotomy dictates venous thromboembolism prophylaxis, and options are found in Table 39-8.
Salpingo-oophorectomy performed via laparotomy typically requires general anesthesia to allow staging of the upper abdomen if malignancy is found. The patient is supine. After anesthesia induction, hair in the planned incision path is clipped if needed; a Foley catheter is inserted; and abdominal preparation is completed. Because of a possible need for hysterectomy if malignancy is found, the vagina is also surgically prepared.
Either a transverse or vertical incision may be used for SO. Clinical factors such as ovarian size and risk of malignancy influence this selection, as discussed on page 926.
Following abdominal entry, cell washings from the pelvis and upper abdomen are collected prior to ovarian manipulation. These are sent for pathologic evaluation if cancer is found. The upper abdomen and pelvis are explored. Peritoneal or omental implants are sampled and sent for intraoperative frozen-section analysis.
A self-retaining retractor such as an O’Connor O’Sullivan or Balfour retractor is placed, and the bowel is packed from the operating field. The affected adnexa is grasped and elevated from the pelvis. If extensive adhesions are found, normal anatomic relationships are restored.
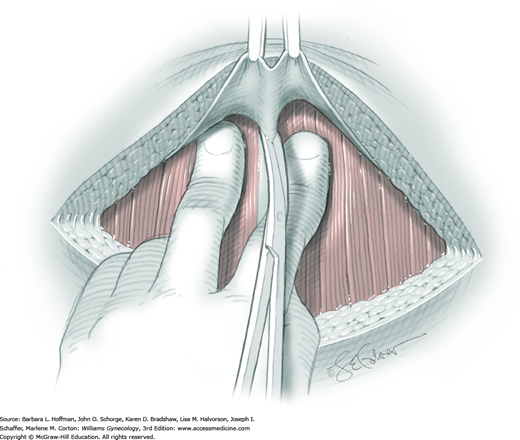
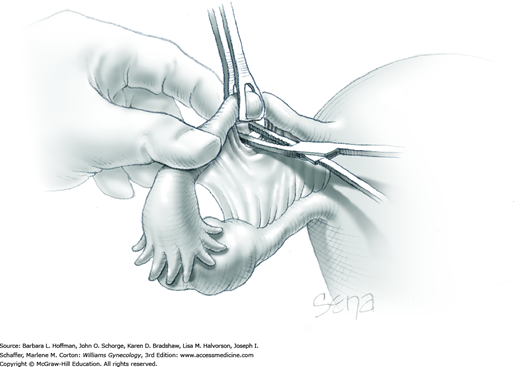
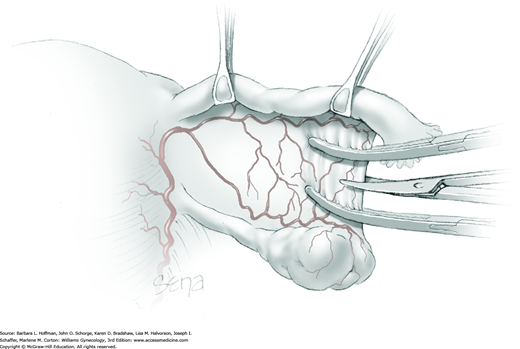
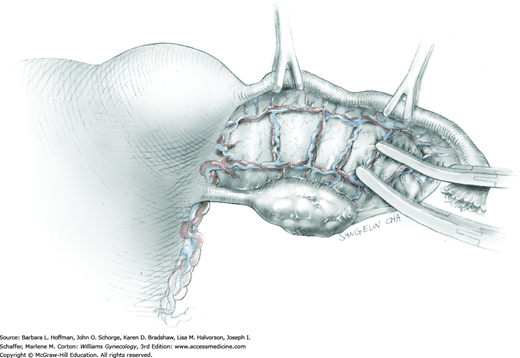
Because of its close proximity to the IP ligament, the ureter is identified prior to clamp placement. In many instances, the ureter is seen beneath the posterior pelvic sidewall peritoneum. Here, it can often be identified as it enters the pelvis and crosses over the common iliac artery bifurcation just medial to the ovarian vessels. In other cases, retroperitoneal isolation of the ureter is required. For this, the peritoneum within the area bounded by the round and IP ligaments and the external iliac vessels is tented with tissue forceps and incised. This first peritoneal incision is extended cephalad toward the pelvic brim (Fig. 43-6.1). The incision also later assists in isolating the IP ligament for ligation. Once this peritoneal window is open, blunt dissection is directed deep, cephalad, and slightly medially through gauzy areolar connective tissue (see Step 6 of abdominal hysterectomy). The ureter is typically found attached to the medial leaf of the incised peritoneum.
The adnexa is lifted from the pelvis and inspected. To isolate the IP ligament, a second peritoneal opening is sharply created with Metzenbaum scissors or electrosurgical blade. It is made in the posterior leaf of the broad ligament below the IP ligament but above the ureter. This incision is extended medially beneath the fallopian tube and uteroovarian ligament and toward the uterus. While remaining parallel to the IP ligament, it is also extended lateral and cephalad towards the pelvic brim. Ideally, the ureter is in view during this entire incision.
As a result of both peritoneal incisions, the IP ligament is isolated. This vascular ligament is then clamped with a Heaney or other sturdy clamp, and the clamp curve faces upward (Fig. 43-6.2). Of note, if SO is performed for cancer risk reduction, the clamp is brought across the IP close to the sidewall. A single Kelly (pean) clamp is placed across the IP at a distance medial to the Heaney clamp. During completion of adnexectomy, this medial clamp prevents “back-bleeding” and is removed with the specimen.
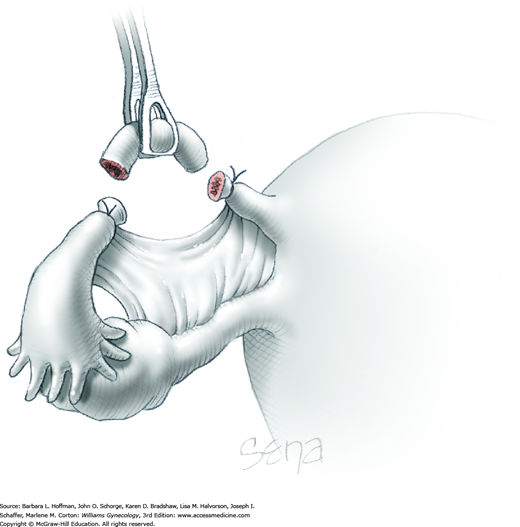
As shown, the ligament is transected between the Heaney and Kelly clamps. To ligate the IP pedicle, a free tie of 0-gauge delayed-absorbable suture is placed around the Heaney clamp. As the knot is secured, this clamp is opened and closed quickly, that is, “flashed.” Next, a transfixing suture is placed around the Heaney clamp (Fig. 40-22). This suture is placed below the clamp yet distal to the first free tie to avoid hematoma formation by needle puncture of ovarian vessels. As this knot is cinched in place, the Heaney clamp is removed.
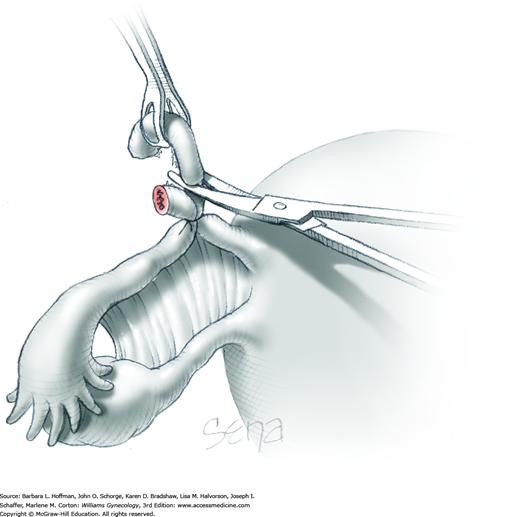
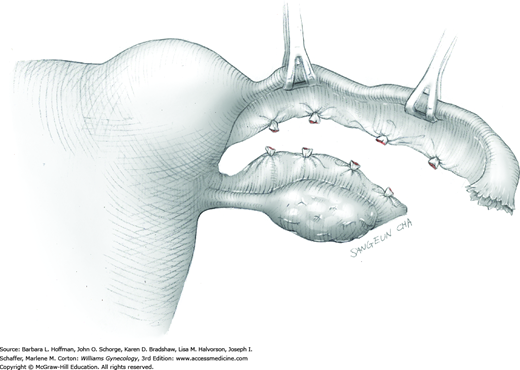
With the adnexa elevated, a Heaney or similar clamp is placed across both the proximal uteroovarian ligament and fallopian tube. It also incorporates some of the mesosalpinx and mesovarium. The clamp’s curve faces the ovary. Next, another clamp enters laterally and is directed medially to close around the remaining mesosalpinx and mesovarium beneath the ovary (Fig. 43-6.3). Again, the clamp curve faces the ovary. Ideally, the tips of both clamps touch beneath the adnexa. Above both of these clamps are stacked second clamps, which lie a distance above their partners and closer to the ovary. Tissue between the stacked clamps is cut with curved Mayo scissors to free the adnexa.
The freed adnexa is removed from the operative site and sent to pathology for evaluation. If malignancy is suspected, an intraoperative frozen section is requested. Tissue within each of the remaining two clamps is individually suture ligated with 0-gauge delayed-absorbable suture.
The retractor and packing sponges are removed from the abdomen. The abdominal incision is then closed as described for vertical or Pfannenstiel incisions.
Patient recovery is similar to that described for laparotomy. In reproductive-aged women, if only one ovary is removed, hormonal and reproductive function is preserved. However, if both are excised, then surgical menopause follows, and hormone replacement is considered (Chap. 22).
Interval Partial Salpingectomy
Interval partial salpingectomy is similar to puerperal midsegment salpingectomy and differs mainly in procedure timing and in abdominal entry. In contrast to postpartum or postabortal sterilization, the term interval designates performance unrelated in time to pregnancy. Accordingly, for most women undergoing interval sterilization, the uterus is small and lies within the confines of the pelvis. Thus, fallopian tubes are reached either laparoscopically or through a low transverse incision.
In general with interval partial salpingectomy, a midtubal segment of fallopian tube is excised, and the severed ends seal by fibrosis and reperitonealization. Commonly used methods of interval sterilization include the Parkland and Pomeroy techniques.
Of tubal sterilization methods, interval partial salpingectomy is infrequently selected for U.S. women who elect sterilization (Peterson, 1996). More commonly, laparoscopic techniques are employed, mainly because of laparoscopy’s postsurgical advantages (Chap. 41). Accordingly, interval partial salpingectomy is typically selected for cases in which laparoscopy may not be indicated. Examples include cases complicated by extensive adhesions, those in which other concurrent pelvic pathology dictates laparotomy, or those in which laparoscopic equipment or surgical skills are lacking. Moreover, new recommendations advocate for risk reducing total salpingectomy when feasible as described on page 939. Thus, the opportunities for laparotomic interval salpingectomy may be few.
As with any sterilization procedure, pregnancy should be excluded prior to the procedure by means of either urine or serum β-human chorionic gonadotropin (hCG) testing. Similarly, to limit the possibility of an early, undetected luteal-phase conceptus, sterilization is ideally performed during the follicular phase of the menstrual cycle, and an effective contraceptive method is used until surgery.
Partial salpingectomy is an effective method of sterilization. Pregnancy rates of less than 2 percent are typical. Failures may result from tubal recanalization or technical errors, such as ligation of the wrong structure.
Tubal sterilization is a safe surgical procedure, and complication rates are below 2 percent (Pati, 2000). Of these, anesthesia complications, organ injury, and wound infection are the most frequent. In addition, although pregnancy is uncommon following sterilization, when pregnancy does occur, the risk of ectopic pregnancy is high and approximates 30 percent (Peterson, 1996; Ryder, 1999). However, because tubal sterilization is highly effective contraception, the overall risk of pregnancy is low, and therefore also is the risk of ectopic pregnancy.
Aside from physical risks, some women experience regret following sterilization. Rates are highest in those 30 years or younger (Curtis, 2006; Hillis, 1999). Accordingly, prior to surgery women are counseled regarding the risk of regret, the permanence of the procedure, and alternative effective long-term contraceptive methods (American College of Obstetricians and Gynecologists, 2011).
Interval partial salpingectomy is usually an outpatient procedure, performed under general or regional anesthesia. Following administration of anesthesia, the patient is placed supine, the abdomen surgically prepared, and the bladder drained.
For most patients, a 4- to 6-cm transverse Pfannenstiel incision is sufficient. Small Richardson or army-navy retractors provide adequate intraabdominal visualization in most cases. A vaginally placed sponge stick or uterine manipulator can elevate the uterus to help bring fallopian tubes into view.
A common reason for sterilization failure is ligation of the wrong structure, usually the round ligament. Identification and isolation of the fallopian tube prior to ligation and submission of tubal segments for pathologic confirmation is therefore required. In some cases, especially those with associated tubal adhesions, this step may be challenging. Lateral extension of the incision may be needed for improved exposure.
Initially, the uterine fundus is identified. At the cornu, insertion of the fallopian tube lies posterior to that of the round ligament, and this orientation can initially guide the surgeon to the correct structure. A primary Babcock clamp is used to elevate the fallopian tube proximally, while a second clamp grasps the tube more distally. The primary clamp is then moved again and is placed distal to the second. The second is then removed and again placed distal to the first. In this manner, the surgeon “marches” down the length of the tube to reach the ampulla and identify fimbria.
At the midpoint of the fallopian tube, an avascular space in the mesosalpinx is identified, and a hemostat is placed directly beneath the tube. The selected site should allow excision of a 2-cm tubal segment that does not incorporate the fimbria. Ligation of the fimbrial portion leads to a greater risk of tubal recanalization and higher failure rates.
The hemostat is bluntly advanced through the mesosalpinx as counterpressure is applied with the index finger. Once advanced through the defect, the hemostat tips are gently opened to expand the aperture (Fig. 43-7.1). The end of a 0-gauge chromic free tie is placed in the tip of the hemostat and pulled through the opening. This is repeated, bringing another tie through the rent. The midsegment is lifted, and the distal suture tied. The second tie is then secured around the proximal fallopian tube.
The Metzenbaum scissor tips are inserted through the mesosalpingeal defect, and the proximal portion of the fallopian tube is cut. A 0.5-cm pedicle is left to ensure that the tube will not slip through its ligature (Fig. 43-7.2). The tube is sharply dissected from the mesosalpinx toward the distal ligature, thereby freeing the tubal segment from the mesosalpinx. The distal part of the segment is excised to leave a 0.5-cm pedicle, and an adequate 2-cm tubal segment is obtained. The pedicles and mesosalpinx are inspected for hemostasis. The procedure is then repeated on the other side. Tubal segments are sent for histologic confirmation of complete transection.
This technique involves grasping and elevating a 2-cm midsegment of tube, ligating the tubal loop with a 2-0 plain catgut suture, and then excising the distal portion of the loop (Fig. 43-7.3). Prompt absorption of the suture following surgery causes the ligated ends to fall away, creating a resulting 2- to 3-cm gap that separates the ends.
The wound is closed as that for other transverse abdominal incisions.
The recovery following minilaparotomy is typically rapid and without complication, and women may resume regular diet and activities as tolerated. Sterilization is immediate following surgery, and intercourse may resume at the patient’s discretion. Aside from regret, the risk of long-term physical or psychologic sequelae is low. Peterson and coworkers (2000) found that women who had undergone tubal sterilization were no more likely than those without this surgery to have menstrual abnormalities. Moreover, interval tubal ligation is unlikely to negatively affect sexual interest or pleasure (Costello, 2002).
Salpingectomy and Salpingostomy
Salpingostomy describes a lengthwise linear incision of the fallopian tube and is usually used to remove intraluminal ectopic pregnancy contents. In contrast, salpingectomy removes the fallopian tube with sparing of the ovary. Indications are varied, and this procedure may be selected for ectopic pregnancy removal, for sterilization, or for hydrosalpinx removal to improve in vitro fertilization success rates. Also, for ovarian cancer prevention, the Society of Gynecologic Oncology (2013) now recommends consideration of salpingectomy in lieu of tubal ligation or at the time of other pelvic surgery. The fallopian tube may be the origin of pelvic serous carcinomas (Chap. 35).
Laparoscopic surgery offers patients the advantages of shorter hospitalizations, quicker recoveries, and less postoperative pain. Accordingly, laparoscopic treatment of ectopic pregnancy is generally preferred. As a result, laparotomic approaches for salpingectomy and salpingostomy are now reserved typically for patients with ruptured ectopic pregnancies who are hemodynamically unstable or in those with contraindications to laparoscopy. For hemoperitoneum, laparotomy offers fast entry into the abdomen for control of bleeding.
Most complications associated with salpingectomy and salpingostomy occur in conjunction with ectopic pregnancies, and the risk of bleeding is prominent. Injury to the ipsilateral ovary, however, is an attendant risk regardless of the indication. In certain cases, if severe, this damage can demand concurrent oophorectomy. Additionally, involvement of the ovary with tubal pathology may necessitate ovarian removal.
If salpingectomy is performed for sterilization, then consenting should mirror that for interval tubal sterilization found in Section 43-7.
Following any surgical treatment of ectopic pregnancy, trophoblastic tissue can persist. Remnant implants typically involve the fallopian tube, but extratubal trophoblastic implants have been found on the omentum and on pelvic and abdominal peritoneum. Peritoneal implants typically measure 0.3 to 2.0 cm and appear as red-black nodules. As expected, the risk of persistent trophoblast tissue is lower with salpingectomy compared with salpingostomy (Farquhar, 2005).
Most, but not all, studies show comparable subsequent fertility rates whether salpingectomy or salpingostomy is performed to treat ectopic pregnancy if the other tube is normal. This discussion is detailed in Chapter 7. Thus, with a healthy contralateral tube, neither salpingostomy nor salpingectomy offers a distinct fertility advantage. However, salpingostomy is considered a preferred option for tubal ectopic pregnancy if there is contralateral tubal disease and a desire for fertility. Unfortunately, in some cases of rupture, the extent of tubal damage or bleeding may limit tubal salvage, and salpingectomy may be required.
If performed for ectopic pregnancy, both salpingectomy and salpingostomy may be associated with substantial bleeding. Baseline complete blood count (CBC) and β-human chorionic gonadotropin (β-hCG) level are obtained. Patients undergo type and screen to establish blood type. Those with significant bleeding also require a type and crossmatch for packed red blood cells and other blood products as indicated. If performed for interval sterilization, then preparation follows that found in Section 43-7.
Salpingectomy and salpingostomy are associated with low infection rates. Accordingly, preoperative antibiotics are usually not required. Laparotomy dictates venous thromboembolism prophylaxis, and options are found in Table 39-8.
In most cases of ectopic pregnancy managed by laparotomy, surgery is an inpatient procedure and requires general anesthesia. For other indications, regional analgesia may be an option. The patient is supine. After anesthesia induction, hair in the planned incision path is clipped if needed; a Foley catheter is placed; and abdominal preparation is completed.
Most salpingectomy or salpingostomy procedures can be managed through a Pfannenstiel incision. However, with a hemodynamically unstable patient and large hemoperitoneum, vertical incision may offer quicker entry.
Once access to the pelvic organs has been achieved, the adnexa is elevated. Distal and proximal Babcock clamps are placed around the fallopian tube and direct the tube away from the uterus and ovary. This extends the mesosalpinx (Fig. 43-8.1).
Beginning at the distal, fimbriated end of the tube, one Kelly clamp or hemostat is placed across a 2-cm-long segment of the mesosalpinx, close to the fallopian tube. The clamp’s curve faces the tube. Another clamp is similarly placed, but lies closer to the ovary. These clamps occlude vessels that traverse the mesosalpinx. Scissors then cut the interposed mesosalpinx.
The severed tissue pedicle that is closer to the ovary is tied with 2-0 or 3-0 gauge delayed-absorbable suture, and the clamp is removed. The clamp closer to the tube remains and leaves with the final specimen. Such clamping, cutting, and ligating are repeated serially, with each clamp incorporating approximately 2 cm of mesosalpinx. Progression is directed from the ampullary end of the fallopian tube toward the uterus.
The last clamp is placed across the proximal mesosalpinx and fallopian tube. Scissors then cut the mesosalpinx and tube and free these from the uterus. This pedicle is similarly ligated.
Surgical steps for salpingostomy mirror those used in laparoscopic salpingostomy and can be reviewed in Section 44-5. To summarize, the affected fallopian tube is elevated with Babcock clamps. At the ectopic pregnancy site, the tube is sharply incised lengthwise on its antimesenteric border. The incision, usually 1 to 2 cm long, varies based on pregnancy size. The products of conception are grasped and gently extracted or are delivered by hydrodissection. Bleeding sites are made hemostatic with electrosurgical coagulation, and the tubal incision is left to heal by secondary intention.
The pelvis is irrigated and rid of blood and tissue debris. The abdominal incision is closed as previously described for vertical or Pfannenstiel incision.
In cases performed for ectopic pregnancy, salpingectomy or salpingostomy represents pregnancy termination. Accordingly, the Rh status of the patient should be evaluated. Administration of 50 or 300 μg (1500 IU) of anti-D immune globulin intramuscularly within 72 hours after pregnancy termination in Rh negative women can dramatically lower the risk of alloimmunization in future pregnancies.
Because of the increased risk of persistent trophoblastic tissue in patients undergoing salpingostomy, serial weekly serum β-hCG levels should be measured until undetectable levels are reached. During this time, contraception should be used to avoid confusion between persistent trophoblastic tissue and a new pregnancy.
For elective sterilization, postoperative instructions follow those for interval tubal sterilization in Section 43-7. For all indications, resumption of activity and diet follow that for laparotomy.
Cornuostomy and Cornual Wedge Resection
Interstitial pregnancy develops in a distensible portion of the tube surrounded by myometrium (Fig. 43-9.1). This location often permits pregnancies to attain greater size than ectopic pregnancy at other sites. Also, uterine rupture at the cornu, where uterine and ovarian arteries anastomose, can lead to brisk and significant hemorrhage. Fortunately, high-resolution sonography, β-hCG testing, and use of established diagnostic criteria have led to earlier diagnosis of interstitial pregnancy. This averts rupture in many circumstances.
In selected cases, this unusual type of ectopic pregnancy may be managed medically, but it is more frequently managed by various surgical techniques. Cornuostomy is analogous to linear salpingostomy for tubal ectopic pregnancy, whereas cornual wedge resection removes the interstitial pregnancy with its surrounding myometrium and fallopian tube (Moawad, 2010). Cornual wedge resection, often performed via laparotomy, has remained a cornerstone of therapy. However, many cases of interstitial pregnancy are now managed laparoscopically (Hwang, 2011). Factors to consider in selecting surgical route and specific procedure include gestational age, presence of rupture, hemodynamic stability, patient’s desire for future fertility, and surgeon’s preference and skill.
This discussion describes a laparotomic approach. However, the principles and surgical steps presented here are applicable to laparoscopic management with only minor modifications.
In some cases, particularly those in which the cornu has ruptured and the woman is hemodynamically unstable, fluid resuscitation and blood transfusion are initiated preoperatively. Further, because there is risk for excessive intraoperative bleeding, a patient is typed and crossmatched for packed red blood cells and other blood products as indicated. A patient is counseled regarding the possible need for blood products, which includes anti-D immune globulin for those with Rh-negative blood. Baseline CBC and β-hCG levels are obtained.
Additional risks include removal of the ipsilateral ovary and the possibility of hysterectomy for uncontrollable bleeding. In the event that the patient has completed her childbearing, tubal ligation or bilateral salpingectomy or rarely even hysterectomy may be acceptable at the time of surgery.
Other than optimizing hemodynamic stability of the patient and ensuring blood availability, no special preparation is required. Prophylactic antibiotics or bowel preparation are generally not required. Laparotomy dictates venous thromboembolism prophylaxis, and options are found in Table 39-8.
Cornual wedge resection and cornuostomy are usually performed under general anesthesia, particularly if cornual rupture is suspected. The patient is supine. After anesthesia induction, hair in the planned incision path is clipped if needed; a Foley catheter is inserted; and abdominal preparation is completed.
Either a transverse or vertical incision may be used depending on the clinical situation as discussed in Section 43-1.
In the absence of cornual rupture and active bleeding, the bowel is packed away to provide adequate exposure of the pelvis. A self-retaining retractor may then be placed. If significant hemoperitoneum is encountered upon abdominal entry, the operator can attempt to remove obscuring blood with suction and laparotomy sponges. Failing this, the surgeon may consider manually elevating the uterus out of the pelvis where it may be inspected for rupture and hemorrhage. The uterus can be compressed between the operator’s thumb and fingers to tamponade bleeding. Two heavy clamps can then be place across the base of the cornu. In rare cases, temporary compression of the aorta may be helpful if bleeding is torrential and poorly controlled.
The location of the ectopic pregnancy is identified. Additional information including presence or absence of rupture, pregnancy size, amount of bleeding, and appearance of the contralateral (unaffected) adnexa is needed before deciding on the exact procedure to perform.
For either cornuostomy or cornual wedge resection, dilute vasopressin (20 units in 30-100 mL of normal saline) may be injected into the myometrium surrounding the interstitial pregnancy to aid hemostasis. Needle aspiration prior to injection is imperative to avoid intravascular injection of this potent vasoconstrictor. The anesthesiologist is concurrently informed of vasopressin injection because a sudden increase in patient blood pressure may follow injection. Blanching at the injection site is expected.
A linear incision is made through the uterine serosa and myometrium overlying the interstitial pregnancy (Fig. 43-9.2). As the incision is carried downward, some products of conception may extrude through the incision (Fig. 43-9.3). Products of conception may be removed by means of blunt, sharp, suction, or hydrodissection (Fig. 43-9.4). Despite vasopressin, bleeding from the myometrium is common and is best managed with electrosurgical coagulation or figure-of-eight stitches with 2-0 gauge absorbable or delayed-absorbable suture.
The myometrial incision is usually closed with absorbable or delayed-absorbable suture in an interrupted or continuous running fashion (Fig. 43-9.5) A gauge of sufficient strength to prevent breakage during muscle approximation is selected, typically 2-0 or 0-gauge. For this, chromic suture may be preferred due to its slight elasticity that provides tensile strength and minimal tissue cutting. Closure may be completed with one layer of sutures or may require two to three layers to aid hemostasis, avert hematoma formation, and reapproximate myometrium. Additionally, some prefer a subserosal closure, similar to a subcuticular running stitch, as a final layer. This theoretically minimizes the amount of exposed suture and thereby limits adhesion formation.
With this approach, the pregnancy, surrounding myometrium, and ipsilateral fallopian tube are excised en bloc. The fallopian tube is removed to avoid future ectopic pregnancy in this tube. Such pregnancies form when the ipsilateral ovary’s eggs are fertilized by sperm that travel out the contralateral tube and are transported by peritoneal fluid to the isolated and ligated tube.
Initially, salpingectomy is completed as described in Section 43-8.1. To summarize, the mesosalpinx is serially clamped and ligated across its length (Fig. 43-9.6). This separates the tube from its mesosalpinx and ipsilateral ovary (Fig. 43-9.7).
Following vasopressin injection, the cornual serosa surrounding the pregnancy is incised with an electrosurgical blade (Fig. 43-9.8). The incision is angled inward as it is deepened. This creates a wedge into the myometrium (Fig. 43-9.9). Hemostasis can be achieved with electrosurgical blade coagulation or with sutures.
The myometrial incision is usually closed in two to three layers with absorbable or delayed-absorbable suture in an interrupted or continuous running fashion. As with cornuostomy, some recommend a final subserosal layer closure. However, depending on the degree of wound tension created by the contracted myometrium, this suture may pull through the serosa, and a simple interrupted or running suture line may be required to approximate the serosa (Fig. 43-9.10).
As noted earlier, there can be cases with rupture and brisk bleeding, in which two clamps are quickly placed across the base of the cornu to halt hemorrhage. In these cases, salpingectomy is similarly completed. Then, the cornual myometrium above these clamps is sharply removed. The myometrium within each clamp is then suture ligated with a transfixing stitch.
After surgery, patient care in general follows that described for laparotomy. As with salpingostomy for treatment of tubal pregnancy, there is increased risk of persistent trophoblastic tissue following cornuostomy. Therefore, serial β-hCG levels are followed postoperatively until a negative test result is obtained. For Rh-negative women, 50 or 300 μg (1500 IU) of anti-D immune globulin is given intramuscularly within 72 hours after pregnancy termination to lower the risk of alloimmunization in future pregnancies. Patients should also be counseled that there is also an increased risk of future ectopic pregnancy in the remaining tube following an interstitial pregnancy. Last, as is the case with other types of uterine surgery such as classical cesarean delivery or myomectomy, the uterine rupture rate in subsequent pregnancies and particularly during labor is increased. For this reason, delivery by cesarean at term before labor onset is generally recommended.
Abdominal Myomectomy
Myomectomy involves surgical removal of leiomyomas from their surrounding myometrium. Indications can include abnormal uterine bleeding, pelvic pain, infertility, and recurrent miscarriage. Hysterectomy is chosen to treat many of these indications. However, myomectomy is often selected by those desiring organ preservation for childbearing or those wishing to avoid hysterectomy.
Myomectomy often requires laparotomy. However, laparoscopic excision may be performed by those with skills in laparoscopic suturing and is described in Section 44-8 (Seracchioli, 2000; Sizzi, 2007).
Because of their influence on pre- and intraoperative planning, leiomyoma size, number, and location are evaluated prior to surgery with sonography, MR imaging, or hysteroscopy (Chap. 9). For example, submucous tumors are more easily removed hysteroscopically (Section 44-14), whereas intramural and serosal types typically require laparotomy or laparoscopy. Leiomyomas may be small and buried within the myometrium. Thus, accurate information as to leiomyoma number and location aids complete excision. Last, multiple large tumors or those that are located in the broad ligament, encroach on the tubal ostia, or involve the cervix may increase the risk of conversion to hysterectomy. Patients are so counseled.
Myomectomy has several risks including significant bleeding and need for transfusion. Moreover, uncontrolled hemorrhage or extensive myometrial injury during tumor removal may force hysterectomy. Fortunately, rates of conversion to hysterectomy during myomectomy are low and range from 0 to 2 percent (Iverson, 1996; LaMorte, 1993; Sawin, 2000). Postoperatively, the risk of pelvic adhesion formation is significant. Also, leiomyomas can recur.
Abnormal uterine bleeding is a common indication for myomectomy. As a result, many women who elect to undergo this surgery are anemic. In addition, significant intraoperative blood loss during myomectomy is possible. Accordingly, attempts to resolve anemia and bleeding prior to surgery are pursued. Toward this goal, oral iron therapy, gonadotropin-releasing hormone (GnRH) agonists, and progesterone antagonists may have benefits (Chap. 9).
In addition to preoperative control of abnormal uterine bleeding, these agents have been shown to significantly decrease uterine volume after several months of use (Benagiano, 1996; Friedman, 1991). Decreased uterine size following treatment may allow a less invasive surgical procedure. For example, myomectomy may be completed through a smaller laparotomy incision or by laparoscopy or hysteroscopy (Lethaby, 2002; Mencaglia, 1993). These agents have also been found to diminish leiomyoma vascularity and uterine blood flow (Matta, 1988; Reinsch, 1994). For this surgery, there is conflicting evidence regarding a final benefit of adhesion prevention (Coddington, 2009; Imai, 2003).
The use of preoperative GnRH agonists, however, may also have disadvantages. Within leiomyomas, GnRH agonists can incite hyaline or hydropic degeneration, which may obliterate the pseudocapsule connective tissue interface between the tumor and the myometrium. Such obliterated cleavage planes may lead to tedious and lengthy tumor enucleation (Deligdisch, 1997). Moreover, studies have shown higher rates of leiomyoma recurrence in women treated with GnRH agonists prior to myomectomy (Fedele, 1990; Vercellini, 2003). Leiomyomas treated with these agents may shrink in volume and be missed during surgical removal.
For these reasons, GnRH agonists are not used routinely in all patients undergoing myomectomy. They can be recommended for preoperative use in women with greatly enlarged uteri or preoperative anemia or in cases in which a decrease in uterine volume would allow a less invasive approach to leiomyoma removal.
Similar to GnRH agonists, the oral progesterone agonists preoperatively shrink myoma volume and diminish menorrhagia (Donnez, 2012a,b). Currently available outside the United States, ulipristal acetate (Esmya) in dosages of 5 mg or 10 mg daily may be used during the 3 months prior to surgery.
The risk of blood transfusion varies among studies and ranges from less than 5 percent to nearly 40 percent (Darwish, 2005; LaMorte, 1993; Sawin, 2000; Smith, 1990). Accordingly, in women with large uteri, especially those with multiple leiomyomas, cell-saver blood scavenger and reuse techniques may be selected (Son, 2014; Yamada, 1997). Indications, benefits, and limitations are discussed fully in Chapter 40.
Also with large leiomyomas, tourniquets or vasopressin may fail to adequately limit bleeding. For these, preoperative uterine artery embolization (UAE) on the morning of surgery may be an effective tool to limit blood loss. And unlike GnRH agonist use, UAE allows tissue planes to be preserved (Chua, 2005; Ngeh, 2004; Ravina, 1995).
Several disadvantages of UAE include risks for subsequent pregnancy complications, collateral ovarian infarction, and formation of uterine synechiae, among others discussed in Chapter 9. Thus, preoperative UAE may best be limited to patients with large uteri in whom excessive blood loss is expected and in those not seeking future pregnancy.
Few studies address the benefits of preoperative antibiotic use. Iverson and coworkers (1996), in their analysis of 101 myomectomy cases, found that although 54 percent of cases received prophylaxis, infectious morbidity was not lowered compared with cases in which antibiotics were not used. However, in cases performed for infertility, because of the potential for tubal adhesions associated with pelvic infection, antibiotic prophylaxis has been advocated (Milton, 2013). For those in whom prophylaxis is planned, selection can follow that for hysterectomy (Table 39-6). For all cases, because the risk of conversion to hysterectomy is present, vaginal preparation immediately prior to surgical draping is warranted.
With myomectomy, the risk of bowel injury is low. Thus, bowel preparation is typically not required unless extensive adhesions are anticipated. Last, laparotomy dictates venous thromboembolism prophylaxis, and options are found in Table 39-8.
Myomectomy performed through a laparotomy incision is typically an inpatient procedure performed under general or regional anesthesia. The patient is supine. After anesthesia induction, hair in the planned incision path is clipped if needed; a Foley catheter is inserted; and abdominal preparation is completed.
The choice of Pfannenstiel incision is typically appropriate for uteri 14-weeks size or smaller (Section 43-2). Larger uteri usually require a midline vertical abdominal incision.
Following abdominal entry, the surgeon inspects the serosal surface to identify leiomyomas to be removed. Additionally, squeezing palpation of the myometrium before and during the surgery will help identify firm buried intramural or submucous leiomyomas.
Tourniquets have been used for years to temporarily occlude blood flow through the uterine arteries. Because the uterus receives collateral flow through the ovarian arteries, some tourniquet techniques include occlusion of both uterine and ovarian vessels. First, bilateral windows are created in the leaves of the broad ligament at the level of the internal cervical os. A Penrose drain or Foley catheter is threaded through the opening to encircle the uterine isthmus. Once in place, the Penrose drain is tied or the ends of the Foley catheter are clamped to compress the uterine vessels. Alternatively, the uterine arteries can be ligated bilaterally (Helal, 2010; Sapmaz, 2003). In combination with uterine artery compression, occlusion of the uteroovarian ligaments or infundibulopelvic ligaments to compress the ovarian arteries has been described (Al-Shabibi, 2009; Taylor, 2005). Large, isthmic, or broad-ligament leiomyomas, however, may limit the use of tourniquets in some.
8-Arginine vasopressin (Pitressin) is a sterile, aqueous solution of synthetic vasopressin. It is effective in limiting uterine blood loss during myomectomy because of its ability to cause vascular spasm and uterine muscle contraction. Compared with placebo, vasopressin injection significantly decreases blood loss during myomectomy (Frederick, 1994). Compared with tourniquet techniques, vasopressin injection has also been associated with either comparable or less intraoperative blood loss, with equally low patient morbidity, and lower myometrial hematoma formation rates (Darwish, 2005; Fletcher, 1996; Ginsburg, 1993).
Each vial of Pitressin is standardized to contain 20 pressor units/mL, and doses used for myomectomy are 20 U diluted in 30 to 100 mL of saline (Frishman, 2009). Vasopressin is typically injected along the planned serosal incision(s). The plasma half-life of this agent is 10 to 20 minutes. For this reason, injection of vasopressin is ideally discontinued 20 minutes prior to uterine repair to allow evaluation of bleeding from myometrial incisions (Hutchins, 1996).
The main risks associated with local vasopressin injection result from inadvertent intravascular infiltration and include transient increases in blood pressure, bradycardia, atrioventricular block, and pulmonary edema (Deschamps, 2005; Tulandi, 1996). For these reasons, patients with a history of cardiovascular disease, cardiomyopathy, congestive heart failure, uncontrolled hypertension, migraine, asthma, and severe chronic obstructive pulmonary disease may not be candidates for vasopressin use. In addition to vasopressin, other less-researched agents for blood loss prevention have been reviewed by Kongnyuy and Wiysonge (2014).
Because of postoperative adhesion formation risks, surgeons ideally minimize the number of serosal incisions and attempt to place incisions on the anterior uterine wall. Tulandi and colleagues (1993) found that posterior wall incisions result in a 94-percent adhesion formation rate compared with a 55-percent rate for anterior incisions.
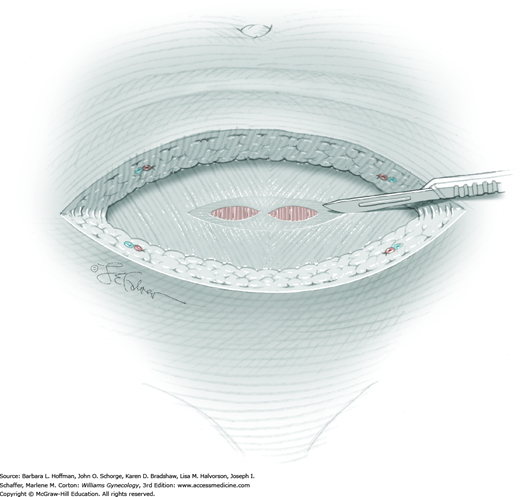
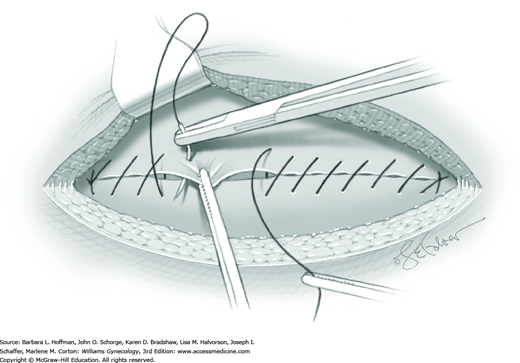
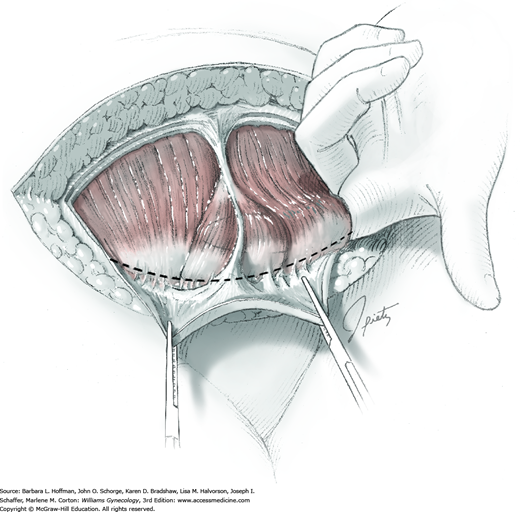
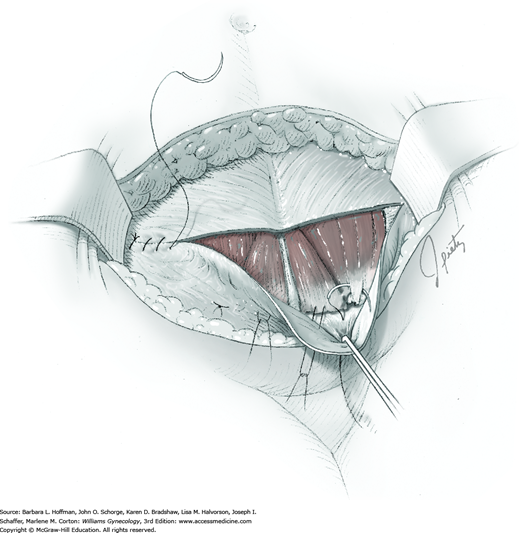
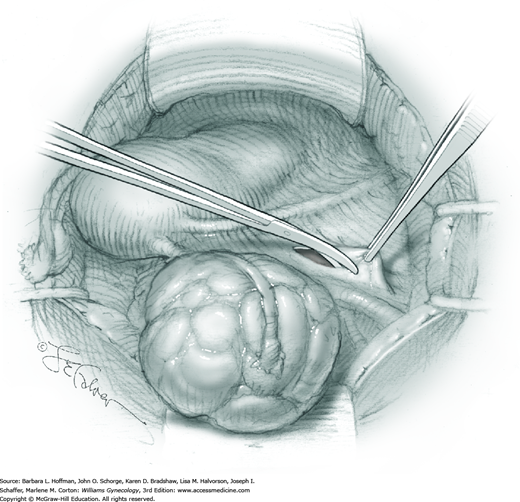
For most patients, a midline vertical uterine incision allows removal of the greatest number of leiomyomas through the fewest incisions. The length should accommodate the approximate diameter of the largest tumor. The incision depth should afford access to all leiomyomas (Fig. 43-10.1). To reach lateral tumors, a surgeon may create lateral myometrial incisions within the initial central incision. However, at times, separate incision may be required to excise tumors. In these instances, a horizontal incision decreases the number of arcuate vessels transected.
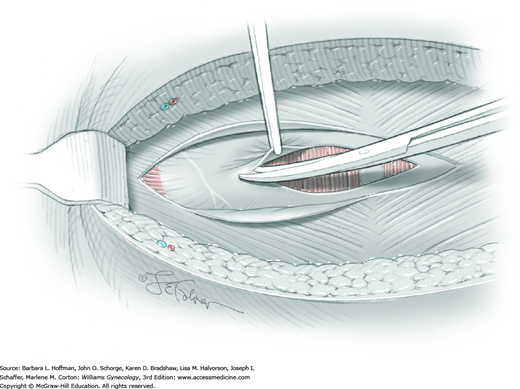
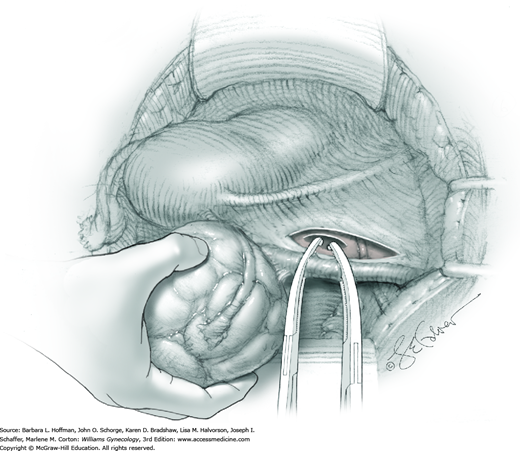
The first leiomyoma is grasped with a Lahey or single-tooth tenaculum (Fig. 43-10.2). Applying traction on the leiomyoma outward and away from the myometrial incision aids in the development of a tissue plane between myometrium and leiomyoma. Sharp and blunt dissection of the pseudocapsule surrounding the leiomyoma frees the tumor from the adjacent myometrium.
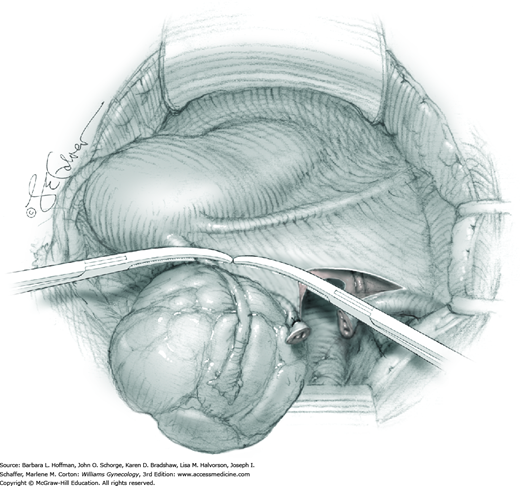
Hemorrhage during myomectomy primarily develops during tumor enucleation and is positively correlated with preoperative uterine size, total weight of leiomyomas removed, and operating time (Ginsburg, 1993). Approximately two to four main arteries feed each leiomyoma and enter the tumor at unpredictable sites. Accordingly, surgeons should watch for these vessels, ligate them prior to transection when possible, and be ready to immediately grasp them with hemostats for ligation or fulguration if lacerated during tumor excision (Fig. 43-10.3).
Smaller, internal incisions into the myometrium may be required to excise all leiomyomas. If the endometrial cavity is entered, it should be closed with a running suture of 4-0 or 5-0 gauge delayed-absorbable suture (Fig. 43-10.4).
After removal of all tumors, redundant serosa may be excised. Smaller internal myometrial incisions are closed first with delayed-absorbable suture (see Fig. 43-10.4). The myometrium is then closed in several layers to improve hemostasis and prevent hematoma formation. A gauge of sufficient strength to prevent breakage during muscle approximation is selected, typically 2-0 to 0-gauge.
Closure of the serosal incision using a running baseball stitch or a subserosal running closure, similar to a running subcuticular closure, may help to limit adhesion formation. For this, 4-0 or 5-0 monofilament, delayed-absorbable suture may be selected. Moreover, absorbable adhesion barriers may help reduce the incidence of adhesion formation following myomectomy (Ahmad, 2015; Canis, 2014; Tinelli, 2011).
After surgery, care in general follows that described for laparotomy. Febrile morbidity of greater than 38.0°C is a common event following myomectomy (Iverson, 1996; Rybak, 2008). Purported causes include atelectasis, myometrial incisional hematomas, and factors released with myometrial destruction. Although fever is common following myomectomy, pelvic infection is not. For example, LaMorte and colleagues (1993) noted only a 2-percent rate of pelvic infection in their analysis of 128 myomectomy cases.
Following myomectomy, there are no clear guidelines as to the timing of pregnancy attempts. Darwish and associates (2005) performed sonographic examinations on 169 patients after myomectomy. Following myometrial indicators, they concluded that wound healing is usually completed within 3 months. There are no clinical trials that address the issue of uterine rupture and therefore route of delivery of pregnancies occurring after myomectomy (American College of Obstetricians and Gynecologists, 2012). Management of these cases requires sound clinical judgment and individualization of care.
Vaginal Myomectomy for Prolapsed Leiomyoma
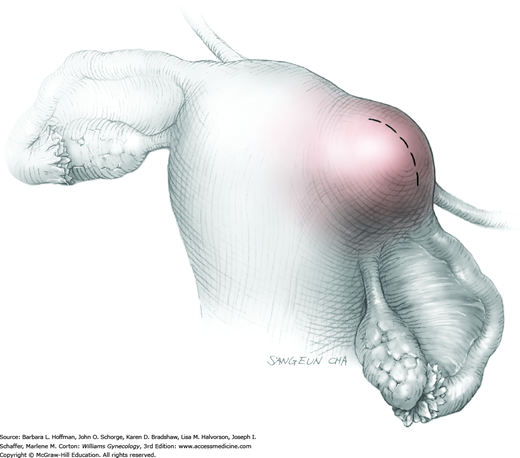
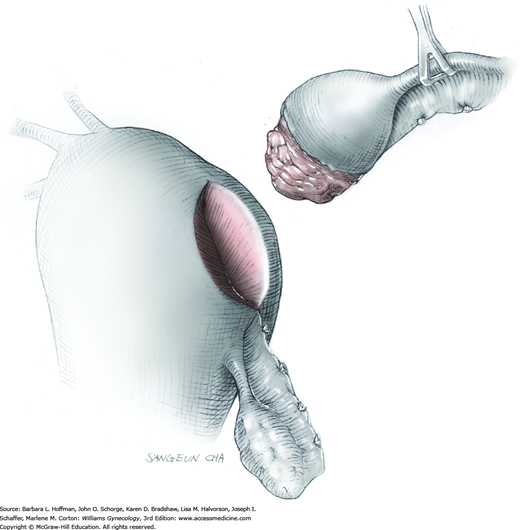
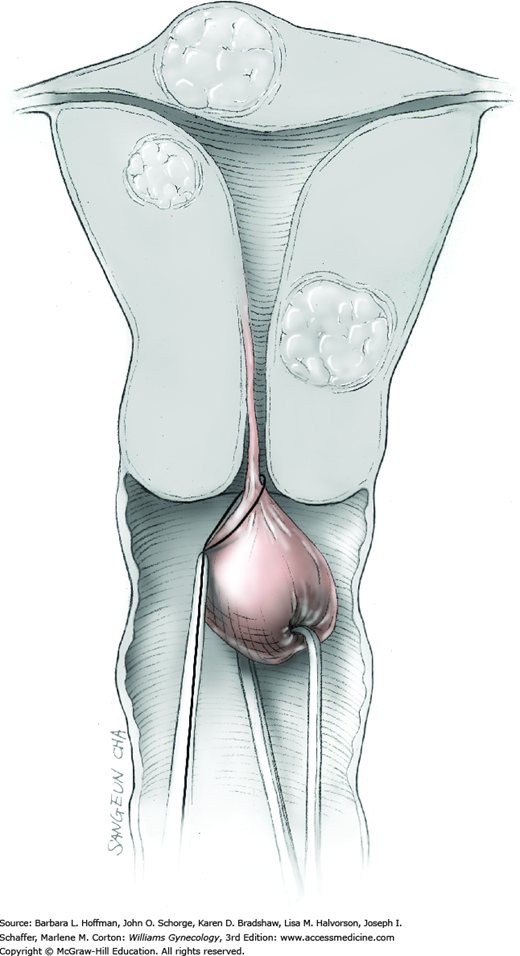
Prolapse of a pedunculated submucosal leiomyoma is an unusual occurrence but certainly not rare. Vaginal myomectomy is usually a relatively simple procedure and is frequently curative for the patient. Myoma and stalk size and patient discomfort are the most important variables for management. With a thin stalk, simply twisting the leiomyoma slowly off its stalk may be sufficient for removal. With larger stalk diameter or greater patient pain, removal in the operating room is typically preferred. Finally, for those with a large obstructive myoma on a thick, short stalk, hysterectomy may be necessary (Caglar, 2005; Golan, 2005).
In many cases, diagnosis of a prolapsed pedunculated submucosal leiomyoma will be obvious, as will the size of the prolapsed leiomyoma. However, as many of these patients present with abnormal uterine bleeding, evaluation for other less obvious causes of abnormal bleeding is appropriate. In other cases, only partial prolapse of a leiomyoma through the cervix may preclude assessment of total leiomyoma and stalk size, or the mass may be of unclear etiology. Accordingly, imaging studies, particularly transvaginal or transabdominal sonography or both, may yield additional information beyond pelvic examination. Specifically, uterine size, shape, and degree of involvement with additional leiomyomas or other pathology can be obtained. Moreover, biopsy of any mass of uncertain etiology is considered, and Tischler biopsy forceps may be selected (Fig. 29-16). If required, Monsel solution can be applied to control bleeding from the biopsy site similar to that following colposcopic biopsy.
Risks with vaginal myomectomy are low. Uncontrollable bleeding and procedural failure are potential complications. Rarely, severing a stalk on great tension may concomitantly resect the attached uterine wall and injure intraabdominal organs. The possibility of hysterectomy and its consequences are also discussed with the patient beforehand. Leiomyoma prolapse recurrence is uncommon but may occur if additional submucosal leiomyomas are present or develop within the uterus.
In an otherwise healthy woman, little preparation is needed for vaginal myomectomy. However, uterine bleeding with leiomyoma prolapse is common, and hypovolemia and acute blood loss anemia are corrected as needed with crystalloid and blood products (Chap. 40). If fever is present and infection of the prolapsed leiomyoma or lower genital tract is suspected, treatment with broad-spectrum antibiotics are initiated prior to vaginal myomectomy. Suitable options are found in Table 39-6. The need for thromboembolism prophylaxis will vary by patient age and anticipated length of surgery as outlined in Table 39-8.
The patient is placed in standard dorsal lithotomy position. Vaginal myomectomy may be performed under general or regional anesthesia, intracervical or paracervical blockade, conscious sedation, or intramuscular analgesia. For those women who are taken to the operating room at our institution, we usually prefer general anesthesia for several reasons. First, hysteroscopy is often done following vaginal myomectomy to further evaluate the uterine cavity and status of the stalk. Secondly, many leiomyomas are bulky and require at least a moderate amount of manipulation and vaginal retraction for removal.
An examination is done once the patient is relaxed to assess the size of the prolapsed leiomyoma; location, length, and thickness of the stalk; and general pelvic anatomy. The vagina is then surgically prepared, and the bladder is drained.
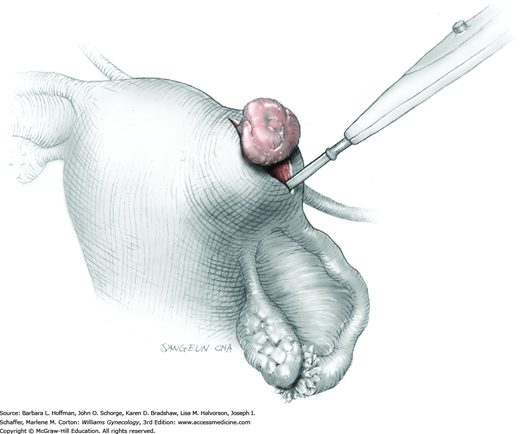
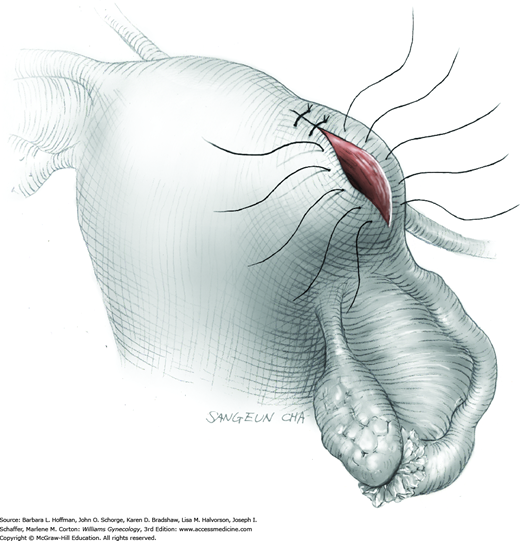
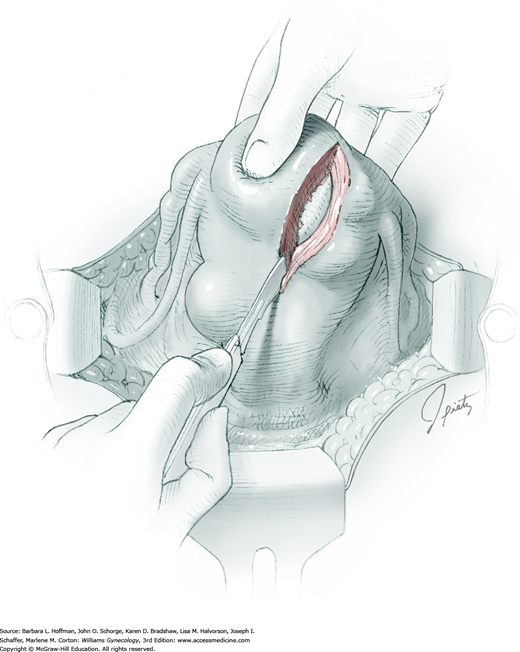
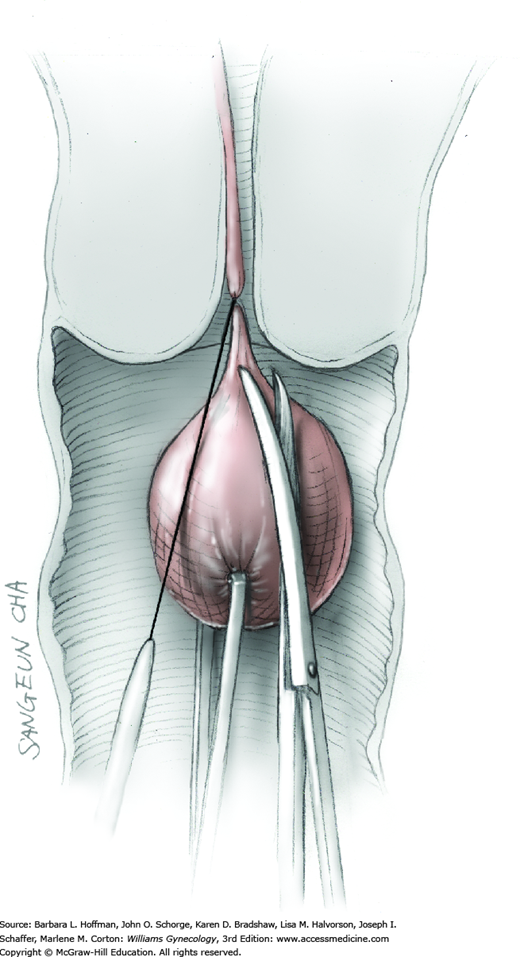
To retract the posterior vaginal wall, an Auvard weighted vaginal speculum is positioned. Heaney retractors are used as needed for sidewall and anterior vaginal wall retraction. The prolapsed leiomyoma is grasped with a tenaculum. Traction is applied on the leiomyoma to allow access to the stalk (Fig. 43-11.1). Excessive traction on the leiomyoma is avoided. This can invert the uterine wall that is attached to the stalk and thereby risk resection of this wall rather than the proximal stalk. In addition, undue traction may avulse the tumor prior to stalk ligation.
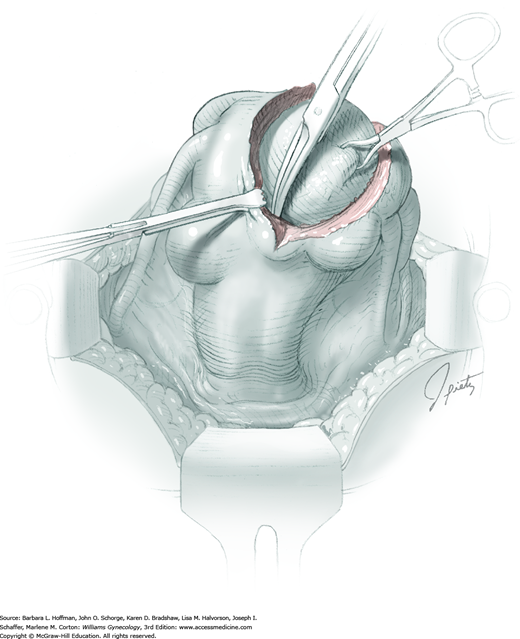
The stalk is doubly ligated with delayed-absorbable suture. Preformed knotted loops with a knot pusher (as used in laparoscopy cases) work well in this setting (Fig. 41-35). With this, the tenaculum is removed to place a loop and then reclamped. In contrast, manual knot tying may be technically difficult given the size of the obstructing leiomyoma, the stalk length (or lack thereof), and the cramped vaginal operating space. In such cases, tips of a Heaney right-angle clamp can be maneuvered past the myoma and then across the stalk.
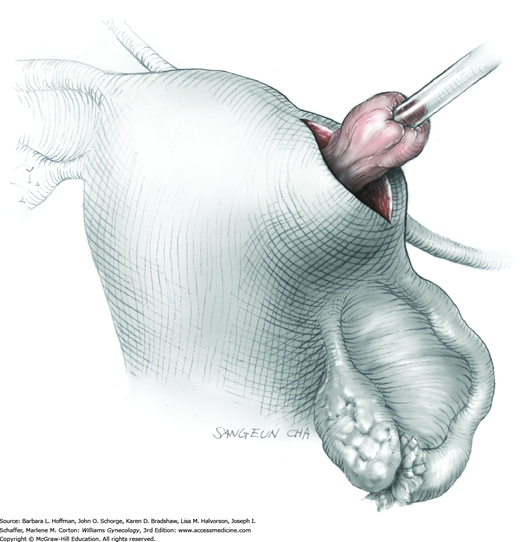
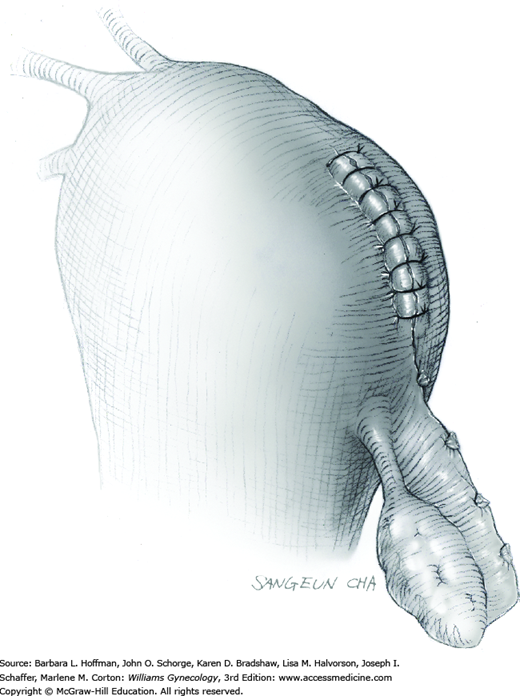
The stalk is then sharply incised at an appropriate point distal to the ligature to prevent the ligature from slipping off (Fig. 43-11.2). With complete stalk transection, the prolapsed leiomyoma is freed for removal, and the ligated stalk retracts into the uterine cavity (Fig. 43-11.3). If a Heaney clamp has been placed, then the stalk is severed, the mass is removed, and a ligature is placed around the proximal stalk. As the suture is tied, the clamp is removed.
As alternatives, the stalk may be incised electrosurgically without ligature placement, or the leiomyoma can be twisted from its stalk if the stalk is not excessively thick. After leiomyoma removal, hysteroscopy may optionally be performed to assess hemostasis and the uterine cavity.
No special care beyond routine postoperative surveillance is necessary following vaginal myomectomy for a pedunculated prolapsed leiomyoma. Regular diet and activities are resumed quickly and can be individualized.
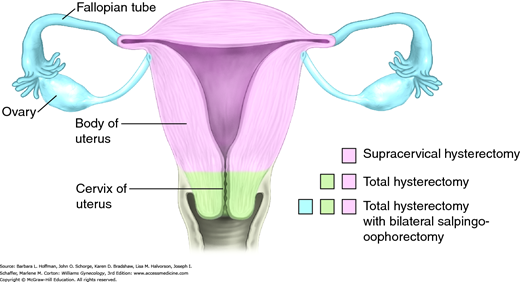
Abdominal Hysterectomy
Hysterectomy is one of the most frequently performed gynecologic procedures, and more than 500,000 women undergo this procedure for benign disease annually in the United States (Jacoby, 2009). Of benign reasons, symptomatic leiomyomas and pelvic organ prolapse are the most frequent, although adenomyosis, endometriosis, chronic pain, and premalignant uterine or cervical disease are also relatively common.
To reach the preoperative diagnosis, testing varies on clinical signs and symptoms and is discussed within the respective chapters covering specific etiologies. Prior to hysterectomy, all patients require cervical cancer screening. With abnormal findings, further evaluation is completed to exclude invasive cancer, which is treated instead with radical hysterectomy or chemoradiation. Similarly, women at risk for endometrial cancer and whose indication includes abnormal bleeding are also usually screened before surgery (Chap. 8). Last, concurrent cervical infection or bacterial vaginosis is sought for preoperative eradication to lower postoperative infection risks.
Hysterectomy may be completed using an abdominal, vaginal, laparoscopic, or robotic approach, and selection is influenced by many factors. For example, shape and size of the uterus and pelvis, surgical indications, presence or absence of adnexal pathology, extensive pelvic adhesive disease, surgical risks, hospitalization and recovery length, hospital resources, and surgeon expertise are all weighed once hysterectomy is planned. Each approach carries distinct advantages and disadvantages, discussed subsequently.
Surgeons usually choose this approach if the uterus is relatively small, extensive adhesions are not anticipated, no significant adnexal pathology is expected, and some degree of pelvic organ descent is present. When this procedure is compared with abdominal hysterectomy, patients usually benefit from faster recovery and from reduced hospital stays, costs, and postoperative pain (Johnson, 2005; Nieboer, 2009).
Despite the advantages of vaginal hysterectomy, most uteri in the United States are removed through an abdominal incision (Jacoby, 2009). Either a transverse or vertical incision may be selected depending on the clinical setting.
Abdominal hysterectomy allows the greatest ability to manipulate pelvic organs. Thus, it may be preferred if large pelvic masses or extensive adhesions are anticipated. Additionally, an abdominal approach affords access to the ovaries if oophorectomy is desired, to the space of Retzius or presacral space if concurrent urogynecologic procedures are planned, or to the upper abdomen for cancer staging. However, for surgeons with advanced skills in minimally invasive surgery (MIS), most of these limitations are overcome, and their indications for abdominal hysterectomy may be few. That said, abdominal hysterectomy typically requires less operating time than laparoscopic or robotic hysterectomy and requires no advanced MIS expertise or instrumentation. Moreover, the Food and Drug Administration (FDA) (2014) has recently discouraged the use of laparoscopic power morcellators due to the potential dispersion of occult cancer cells. While data are being collected regarding this risk, many surgeons and patients may forego MIS hysterectomy for larger uteri, and thus rates of abdominal hysterectomy may increase.
Disadvantages of abdominal hysterectomy include longer patient recovery and hospital stays, increased incisional pain, and greater risk of postoperative fever and wound infection (Marana, 1999; Nieboer, 2009). Additionally, compared with a vaginal approach, abdominal hysterectomy is associated with greater risk for ureteral injury, but lower rates of bladder injury (Frankman, 2010; Gilmour, 2006).
Selected more and more frequently, this hysterectomy group uses laparoscopic techniques to complete some or all steps of hysterectomy, and specific definitions are provided in Chapter 44 (Turner, 2013). Although criteria vary depending on surgeon skill, this approach is often selected if the uterus is not excessively large, extensive adhesions are not expected, and some limitation deters vaginal hysterectomy alone. Patient recovery, hospital stays, and postoperative pain scores are comparable with those of vaginal hysterectomy, but a laparoscopic approach allows greater visualization and access to the abdomen and pelvis. This may be advantageous if oophorectomy is planned or if adhesive disease or bleeding is encountered. However, laparoscopy typically requires longer operating times, expensive equipment, and MIS expertise. In addition, in most studies, laparoscopic hysterectomy has been associated with greater rates of ureteral injury than either abdominal or vaginal hysterectomy (Frankman, 2010; Gilmour, 2006; Mamik, 2014).
If all factors are equal, vaginal hysterectomy should be considered. However, with large pelvic masses or large uteri, with risk of gynecologic cancer, with extensive adhesions, or with poor uterine descent, either abdominal or laparoscopic hysterectomy may be required. Of note, surgical expertise is factored into the decision and strongly dictates the approach selected.
Prior to hysterectomy, the decision to concurrently remove the cervix is discussed with the patient. Hysterectomy may include removal of the uterus and cervix, termed total hysterectomy, or may involve only the uterine corpus, called supracervical hysterectomy (SCH) (Fig. 43-12.1). The term subtotal hysterectomy is ambiguous and is not a preferred term.
Most hysterectomies performed are total, but SCH may be selected preoperatively. For example, SCH is purported to reduce the risk of mesh erosion at the cuff if concurrent hysterectomy and sacrocolpopexy are planned (Osmundsen, 2012; Tan-Kim, 2011). At one point, SCH was also suggested to improve urinary, bowel, or sexual function compared with total abdominal hysterectomy. But, several studies have shown no short- or long-term differences in these functions between total abdominal or supracervical hysterectomy (Learman, 2003; Lethaby, 2012; Thakar, 2002). Frequently, SCH may be an intraoperative decision during cases in which excision of the cervix risks increased bleeding, surrounding organ damage, or increased operating time.
As a disadvantage, 10 to 20 percent of women following SCH will still note cyclic vaginal bleeding, presumably from retained isthmic endometrium in the cervical stump. Procedures that ablate or core out the endocervical canal can help prevent this complication (Schmidt, 2011). Also, pelvic organ prolapse may develop (Hilger, 2005). For either complication, cervical stump excision, termed trachelectomy, may be required. Last, critics noted the persistent risk for cancer in the conserved stump. However, the risk for cervical cancer in these women is comparable to that in women without hysterectomy. Moreover, the prognosis for cervical stump cancer mirrors that in women with a complete uterus (Hannoun-Levi, 1997; Hellstrom, 2001).
In sum, SCH alone offers no distinct long-term advantages compared with total abdominal hysterectomy (American College of Obstetricians and Gynecologists, 2013b). The risk of persistent bleeding following surgery may deter many women and clinicians from its use. Moreover, although data are limited, trachelectomy following SCH may be surgically challenging due to scarring of bowel or bladder to the stump. Despite these disadvantages, if concurrent sacrocolpopexy is planned, SCH may lower mesh erosion rates. However, current data for this are limited and retrospective, and future research is needed.
For most women with indications, hysterectomy is a safe and effective treatment that typically leads to an improved postoperative quality of life and psychological outcome (Hartmann, 2004; Kuppermann, 2013). However, pelvic organs may be injured during surgery, and vascular, bladder, ureteral, and bowel injury are most commonly cited. Accordingly, these and the risks of wound infection, blood loss, and transfusion are discussed with the patient before surgery. Infrequently, unintended adnexectomy may be required, and if bilateral, will create iatrogenic menopause. Importantly, patients should understand the sterilizing effects of hysterectomy.
Hysterectomy is frequently performed with other operations. Pelvic reconstructive surgeries and bilateral salpingo-oophorectomy (BSO) or salpingectomy are among the most frequent.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree