Introduction
The indications for delivery in the second trimester of pregnancy can be the result of obstetrical complications necessitating delivery or pregnancy termination of prenatally diagnosed anomalous fetuses. In such cases, there may be a need for cervical ripening methods. It is noteworthy that 7% of all pregnancy terminations are performed at 14-20 weeks and 1.3% at or >21 weeks’ gestation. Given that a third of all pregnancies are delivered by cesarean in the United States, the number of patients with a prior cesarean delivery (CD) who require a cervical ripening agent in the second trimester of pregnancy is expected to increase.
Different methods of cervical ripening have been used in the second trimester of pregnancy in patients with existing uterine scar including mechanical methods (ie, laminaria or cervical dilators) or medical methods (ie, synthetic prostaglandins). The purpose of these methods is to achieve an expeditious delivery without significant morbidity. However, one rare but well-described serious complication of cervical ripening methods is uterine rupture. Thus, the clinician has to balance the benefit of achieving vaginal delivery in an expeditious manner vs the risk of uterine rupture or any other maternal complications. The efficacy and safety of cervical ripening agents has been extensively studied in the third trimester and in women without a history of CD but much less is known regarding the efficacy vs risks in using these agents in the second trimester in patients with a prior CD.
We undertook a systematic review and metaanalysis to evaluate the efficacy and safety of different cervical ripening agents in the second trimester of pregnancy in patients with previous CD.
Materials and Methods
Identification of studies
This metaanalysis included studies addressing safety and efficacy of cervical ripening methods in the second trimester in patients with ≥1 previous CD. A systematic review of English-language articles was performed using PubMed, EMBASE, CINAHL, LILACS, Google Scholar, and clinicaltrials.gov and by identifying studies cited in the references of published articles. Search terms included “cesarean,” “second trimester, pregnancy termination,” “misoprostol,” “dilation and evacuation,” “dinoprostone,” “PGE2 analogues,” “Foley catheter,” “balloon,” “laminaria,” “hypertonic saline,” “mifepristone,” “PG analogues,” “PGF2α,” “synthetic dilators,” “oxytocin,” “hysterotomy,” and combinations of these. Articles were included from January 1983 through May 2015.
Eligibility criteria
Studies were included for review if data were available regarding efficacy and safety of cervical ripening methods in patients with previous CD. Case reports and case series with <5 cases were excluded. Abstracts and poster presentations were included for review only if they included the aforementioned relevant information. We included both descriptive studies and studies comparing efficacy and safety of different ripening agents between patients with previous CD and those with no uterine scar.
Study selection
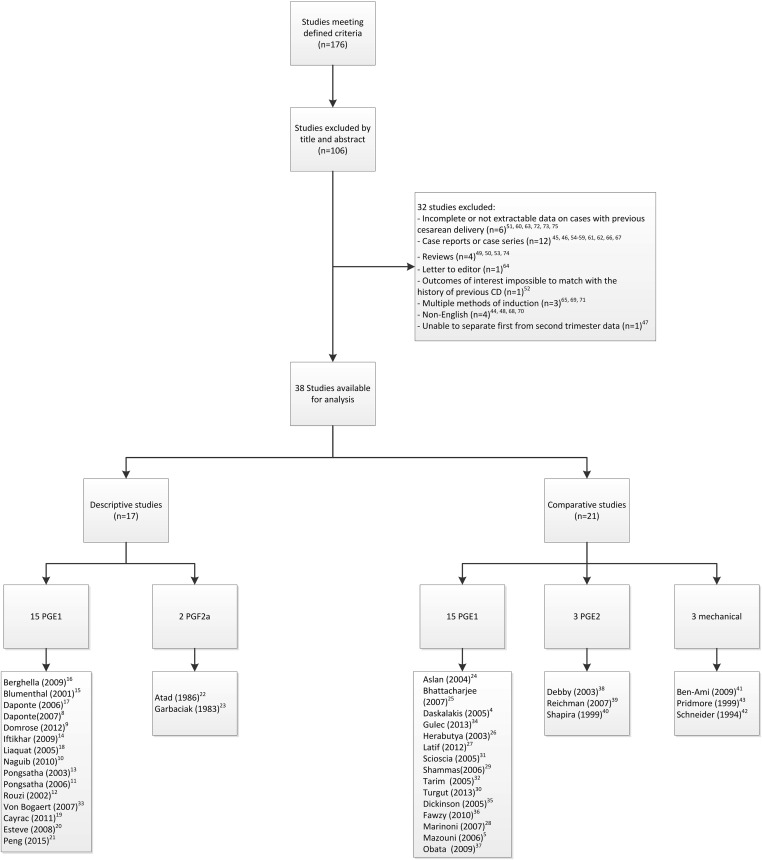
Two authors (M.A. and J.A.L.) were involved in identifying the eligible manuscripts; 176 were initially identified, of which 106 were excluded, after screening the title and abstract, as not being relevant to the aims of the metaanalysis. The texts of the remaining 70 manuscripts were fully reviewed, from which case reports or case series with <5 patients (n = 12), reviews (n = 4), and a letter to the editor (n = 1) were further excluded. We also excluded non-English-language articles (n = 4) because it has been shown that exclusion of such articles has little effect on summary treatment estimates. Additionally, studies where the outcomes of interest was impossible to match with the history of CD (n = 1) or studies with no information or incomplete or not extractable information on cases with previous CD or studies which included small number of cases with previous CD were excluded (n = 6). Articles where the patients within the study group had received multiple ripening methods were excluded on the basis that conclusions could not be drawn for each ripening method separately (n = 3) ( Figure 1 ). Also 1 study, which did not separate first- from second-trimester cases, was excluded, as we could not isolate the second-trimester termination cases. This selection process resulted in 38 studies that fit our inclusion criteria, all of which were reviewed by 1 author (M.A.). In cases of uncertainty regarding inclusion or exclusion, 2 other authors were consulted (C.V.A. and A.M.V.).
Data collection process
Information regarding the type of study; country of origin; year the study was conducted; ripening agent used; gestational age; dose of ripening agent and the protocol used; mode of delivery; duration of delivery; and complications such as uterine rupture, blood transfusion requirement, endometritis, retained placental tissue, and analgesia were collected. When the range of gestational age was not clearly specified, we allocated the studies to second-trimester group according to mean or median gestational age (<28 weeks for second trimester). When prostaglandins were used as ripening agent (with or without oxytocin), this was defined as the main agent. The only exception was when 1 dose of prostaglandin was given prior to dilation and evacuation. These studies were classified under mechanical methods because mechanics was the final main method of termination.
Primary and secondary outcomes
The primary outcome in terms of efficacy of ripening agents was the proportion of patients achieving vaginal delivery (primary measure) as well as vaginal delivery within 24 hours (secondary measure). Safety was assessed as a secondary outcome by the risk of uterine rupture (primary measure) and complications such as retained placental products, blood transfusion requirement, and endometritis, if available (secondary measures). In determining uterine rupture, we grouped true uterine rupture and silent uterine rupture (or dehiscence) together, since silent rupture can be considered as a “near miss.” In addition, it was not always possible to separate out the patients with uterine rupture from those with silent uterine rupture (dehiscence) because the authors did not always distinguish between these 2 conditions. The risk of uterine rupture was assessed overall for patients with ≥1 CD and also in the subgroups with only 1 and ≥2 prior CD if the data were available.
Data synthesis
From descriptive studies we collected descriptive statistics regarding the rate of the outcome in women with a previous CD. Summary measures reported in comparative studies included the risk difference and risk ratio (RR) with 95% confidence intervals (CI) comparing the risk of the outcome in the group with a previous CD to the risk in the group without a previous CD. Risk differences and RR were computed in Review Manager v5.3 (Nordic Cochrane Centre, Copenhagen, Denmark) via the inverse variance method in a random effects analysis. In the absence of heterogeneity across studies, the random effects model will match a fixed effects analysis. In the presence of heterogeneity across studies, this method produces wider CI, and so is more conservative in its estimates of the risk difference and RR. Between-study heterogeneity was assessed based on the I 2 measure, and substantial heterogeneity was considered to be an I 2 >50%.
Assessment of risk of bias
Each study was evaluated for potential bias based on 6 measures. These included representativeness of the population, ascertainment of the exposure, assessment of the outcomes, blinding of the investigators to the exposure, incomplete outcome data (loss to follow-up), and control for confounders. Each of these 6 measures were rated on a 3-level color scale with green denoting that the criterion was met, red denoting that the criterion was not met, and orange denoting an uncertain status. For the 6 criteria, studies received a red rating if the study were conducted in a way that could introduce bias or decrease generalizability. For example, single-center studies received a red rating for being representative of the population, because it is possible that patients at a single institution are more similar to each other and not necessarily representative of all patients in the population. Studies received a rating of green for a bias criterion if there was little or no concern that the study methods introduced bias. For example, for the criterion of incomplete outcome data, studies received a green rating when none of the outcome data were missing for the primary outcome of vaginal delivery.
Materials and Methods
Identification of studies
This metaanalysis included studies addressing safety and efficacy of cervical ripening methods in the second trimester in patients with ≥1 previous CD. A systematic review of English-language articles was performed using PubMed, EMBASE, CINAHL, LILACS, Google Scholar, and clinicaltrials.gov and by identifying studies cited in the references of published articles. Search terms included “cesarean,” “second trimester, pregnancy termination,” “misoprostol,” “dilation and evacuation,” “dinoprostone,” “PGE2 analogues,” “Foley catheter,” “balloon,” “laminaria,” “hypertonic saline,” “mifepristone,” “PG analogues,” “PGF2α,” “synthetic dilators,” “oxytocin,” “hysterotomy,” and combinations of these. Articles were included from January 1983 through May 2015.
Eligibility criteria
Studies were included for review if data were available regarding efficacy and safety of cervical ripening methods in patients with previous CD. Case reports and case series with <5 cases were excluded. Abstracts and poster presentations were included for review only if they included the aforementioned relevant information. We included both descriptive studies and studies comparing efficacy and safety of different ripening agents between patients with previous CD and those with no uterine scar.
Study selection
Two authors (M.A. and J.A.L.) were involved in identifying the eligible manuscripts; 176 were initially identified, of which 106 were excluded, after screening the title and abstract, as not being relevant to the aims of the metaanalysis. The texts of the remaining 70 manuscripts were fully reviewed, from which case reports or case series with <5 patients (n = 12), reviews (n = 4), and a letter to the editor (n = 1) were further excluded. We also excluded non-English-language articles (n = 4) because it has been shown that exclusion of such articles has little effect on summary treatment estimates. Additionally, studies where the outcomes of interest was impossible to match with the history of CD (n = 1) or studies with no information or incomplete or not extractable information on cases with previous CD or studies which included small number of cases with previous CD were excluded (n = 6). Articles where the patients within the study group had received multiple ripening methods were excluded on the basis that conclusions could not be drawn for each ripening method separately (n = 3) ( Figure 1 ). Also 1 study, which did not separate first- from second-trimester cases, was excluded, as we could not isolate the second-trimester termination cases. This selection process resulted in 38 studies that fit our inclusion criteria, all of which were reviewed by 1 author (M.A.). In cases of uncertainty regarding inclusion or exclusion, 2 other authors were consulted (C.V.A. and A.M.V.).
Data collection process
Information regarding the type of study; country of origin; year the study was conducted; ripening agent used; gestational age; dose of ripening agent and the protocol used; mode of delivery; duration of delivery; and complications such as uterine rupture, blood transfusion requirement, endometritis, retained placental tissue, and analgesia were collected. When the range of gestational age was not clearly specified, we allocated the studies to second-trimester group according to mean or median gestational age (<28 weeks for second trimester). When prostaglandins were used as ripening agent (with or without oxytocin), this was defined as the main agent. The only exception was when 1 dose of prostaglandin was given prior to dilation and evacuation. These studies were classified under mechanical methods because mechanics was the final main method of termination.
Primary and secondary outcomes
The primary outcome in terms of efficacy of ripening agents was the proportion of patients achieving vaginal delivery (primary measure) as well as vaginal delivery within 24 hours (secondary measure). Safety was assessed as a secondary outcome by the risk of uterine rupture (primary measure) and complications such as retained placental products, blood transfusion requirement, and endometritis, if available (secondary measures). In determining uterine rupture, we grouped true uterine rupture and silent uterine rupture (or dehiscence) together, since silent rupture can be considered as a “near miss.” In addition, it was not always possible to separate out the patients with uterine rupture from those with silent uterine rupture (dehiscence) because the authors did not always distinguish between these 2 conditions. The risk of uterine rupture was assessed overall for patients with ≥1 CD and also in the subgroups with only 1 and ≥2 prior CD if the data were available.
Data synthesis
From descriptive studies we collected descriptive statistics regarding the rate of the outcome in women with a previous CD. Summary measures reported in comparative studies included the risk difference and risk ratio (RR) with 95% confidence intervals (CI) comparing the risk of the outcome in the group with a previous CD to the risk in the group without a previous CD. Risk differences and RR were computed in Review Manager v5.3 (Nordic Cochrane Centre, Copenhagen, Denmark) via the inverse variance method in a random effects analysis. In the absence of heterogeneity across studies, the random effects model will match a fixed effects analysis. In the presence of heterogeneity across studies, this method produces wider CI, and so is more conservative in its estimates of the risk difference and RR. Between-study heterogeneity was assessed based on the I 2 measure, and substantial heterogeneity was considered to be an I 2 >50%.
Assessment of risk of bias
Each study was evaluated for potential bias based on 6 measures. These included representativeness of the population, ascertainment of the exposure, assessment of the outcomes, blinding of the investigators to the exposure, incomplete outcome data (loss to follow-up), and control for confounders. Each of these 6 measures were rated on a 3-level color scale with green denoting that the criterion was met, red denoting that the criterion was not met, and orange denoting an uncertain status. For the 6 criteria, studies received a red rating if the study were conducted in a way that could introduce bias or decrease generalizability. For example, single-center studies received a red rating for being representative of the population, because it is possible that patients at a single institution are more similar to each other and not necessarily representative of all patients in the population. Studies received a rating of green for a bias criterion if there was little or no concern that the study methods introduced bias. For example, for the criterion of incomplete outcome data, studies received a green rating when none of the outcome data were missing for the primary outcome of vaginal delivery.
Results
Study selection and characteristics
In all, 38 observational studies met the inclusion criteria. There were no randomized controlled trials. Seventeen studies were descriptive without a comparison group (563 patients) and 21 studies compared the efficacy and safety of cervical ripening agents between patients with previous CD and those with no uterine scar (8419 patients). The individual characteristics of the descriptive and comparative studies are outlined in Tables 1 and 2 .
| Study (year) | Country | Duration | Total no. of subjects | Method of induction of labor | Outcomes |
|---|---|---|---|---|---|
| Berghella et al (2009) | United States | 1998 through 2004 | 17 | PGE1 | Vaginal delivery rate, uterine rupture, blood transfusion |
| Blumenthal and Medina (2001) | United States | 1997 through 2000 | 10 | PGE1 | Vaginal delivery rate, uterine rupture, endometritis, blood transfusion |
| Daponte et al (2006) | South Africa | 1997 through 2000 | 85 | PGE1 | Vaginal delivery rate, uterine rupture, blood transfusion |
| Daponte et al (2007) | South Africa | 3 y | 21 | PGE1 | Vaginal delivery rate, uterine rupture, retained products of conception |
| Domröse et al (2012) | Germany | 2005 through 2009 | 100 | PGE1 | Vaginal delivery rate, uterine rupture, blood transfusion |
| Iftikhar and Burney (2009) | Pakistan | 2007 through 2009 | 50 | PGE1 | Vaginal delivery rate, uterine rupture, retained products of conception |
| Liaquat et al (2006) | Pakistan | 2003 through 2005 | 5 | PGE1 | Vaginal delivery rate, uterine rupture |
| Naguib et al (2010) | Egypt | January 2008 through August 2008 | 50 | PGE1 | Vaginal delivery rate, uterine rupture, blood transfusion |
| Pongsatha and Tongsong (2003) | Thailand | Not reported | 21 | PGE1 | Vaginal delivery rate, uterine rupture, retained products of conception |
| Pongsatha and Tongsong (2006) | Thailand | 2003 through 2005 | 17 | PGE1 | Vaginal delivery rate, uterine rupture, blood transfusion |
| Rouzi (2003) | Saudi Arabia | 1998 through 2002 | 10 | PGE1 | Vaginal delivery rate, uterine rupture |
| van Bogaert (2008) | South Africa | 9 mo (year unspecified) | 18 | PGE1 | Uterine rupture |
| Cayrac et al (2011) | France | 2000 through 2008 | 67 | PGE1, mifepristone, laminaria | Vaginal delivery rate, uterine rupture, endometritis, blood transfusion |
| Esteve et al (2008) | Spain | 2003 through 2007 | 17 | PGE1, mifepristone | Vaginal delivery rate, uterine rupture |
| Peng et al (2015) | China | 2006 through 2013 | 33 | PGE1, mifepristone | Vaginal delivery rate, uterine rupture |
| Atad et al (1986) | Israel | Not reported | 13 | PGF2α | Vaginal delivery rate, uterine rupture, blood transfusion |
| Garbaciak and Benzie (1983) | Canada | 1972 through 1979 | 38 | PGF2α, hypertonic saline, laminaria | Vaginal delivery rate, uterine rupture, endometritis, retained products of conception |
| Study (year) | Country | Duration | Design | Total no. of subjects | Method of induction of labor | Outcomes |
|---|---|---|---|---|---|---|
| Aslan et al (2004) | Turkey | 1999 through 2002 | Retrospective cohort | 91 | PGE1 | Vaginal delivery rate, uterine rupture |
| Bhattacharjee et al (2007) | India | 2003 through 2006 | Retrospective cohort | 160 | PGE1 | Vaginal delivery rate, uterine rupture, blood transfusion, endometritis, retained products of conception |
| Daskalakis et al (2005) | Greece | 1997 through 2002 | Retrospective cohort | 324 | PGE1 | Vaginal delivery rate, uterine rupture, retained products of conception, endometritis |
| Güleç et al (2013) | Turkey | 2007 through 2010 | Retrospective cohort | 279 | PGE1 | Vaginal delivery rate, uterine rupture |
| Herabutya et al (2003) | Thailand | 1996 through 2002 | Prospective cohort | 584 | PGE1 | Vaginal delivery rate, uterine rupture, retained products of conception |
| Latif et al (2012) | Egypt | 2010 through 2012 | Prospective cohort | 210 | PGE1 | Vaginal delivery rate, uterine rupture, retained products of conception, blood transfusion |
| Marinoni et al (2007) | Italy | 1998 through 2005 | Retrospective cohort | 429 | PGE1 | Vaginal delivery rate, uterine rupture, blood transfusion |
| Scioscia et al (2007) | Italy | 2000 through 2005 | Retrospective cohort | 423 | PGE1 | Vaginal delivery rate, uterine rupture, blood transfusion |
| Shammas and Momani (2006) | Jordan | 2000 through 2004 | Prospective cohort | 520 | PGE1 | Vaginal delivery rate, uterine rupture, retained products of conception, blood transfusion |
| Tarim et al (2005) | Turkey | 1998 through 2004 | Prospective cohort | 57 | PGE1 | Vaginal delivery, uterine rupture |
| Turgut et al (2013) | Turkey | 2009 through 2012 | Retrospective cohort | 219 | PGE1 | Vaginal delivery rate, uterine rupture, blood transfusion |
| Dickinson (2005) | Australia | 1997 through 2004 | Retrospective cohort | 720 | PGE1, Foley | Vaginal delivery rate, blood transfusion, retained products of conception |
| Fawzy and Abdel-Hady (2010) | Egypt | 2006 through 2009 | Prospective cohort | 138 | PGE1, Foley | Vaginal delivery rate, uterine rupture, blood transfusion, retained products of conception |
| Mazouni et al (2006) | France | 2000 through 2004 | Retrospective cohort | 252 | PGE1, mifepristone, PGE2 | Vaginal delivery rate, uterine rupture, retained products of conception, blood transfusion |
| Obata-Yasuoka et al (2009) | Japan | 1999 through 2006 | Retrospective cohort | 195 | PGE1, laminaria | Vaginal delivery rate, uterine rupture, blood transfusion |
| Debby et al (2003) | Israel | 1987 through 2000 | Retrospective cohort | 261 | PGE2 | Vaginal delivery rate, uterine rupture, retained products of conception |
| Reichman et al (2007) | Israel | 1999 through 2004 | Retrospective cohort | 375 | PGE2 | Vaginal delivery rate, uterine rupture, blood transfusion |
| Shapira et al (1999) | Israel | 1992 through 1997 | Retrospective cohort | 282 | PGE2 | Vaginal delivery rate, uterine rupture, blood transfusion |
| Ben-Ami et al (2009) | Israel | 2002 through 2008 | Retrospective cohort | 636 | Laminaria, dilation and evacuation | Vaginal delivery rate, uterine rupture, blood transfusion, retained products of conception |
| Pridmore and Chambers (1999) | South Australia | 1992 through 1998 | Retrospective and prospective cohort | 1869 | Laminaria, dilation and evacuation, PGE1 | Vaginal delivery rate, uterine rupture |
| Schneider et al (1994) | Israel | 1978 through 1993 | Retrospective cohort | 1064 | Laminaria, dilation and evacuation | Vaginal delivery rate, uterine rupture, blood transfusion |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


