TERMINOLOGY
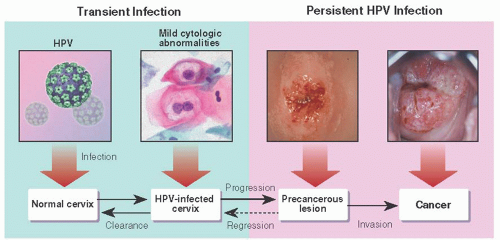
Although carcinoma in situ became well recognized as a full-thickness epithelial change without stromal invasion, the terminology and clinical significance of adjacent, less-than-full-thickness atypia was uncertain. In 1956, Reagan and Hamonic introduced the term dysplasia to designate these cervical epithelial abnormalities that were characterized by cytologic atypia, increased mitotic activity, and loss of polarity, graded as mild, moderate, and severe dysplasia. The poor reproducibility of the distinction between CIS and severe dysplasia and the clear premalignant nature of the latter led Ralph Richart in 1969 to propound the concept of CIN. CIN 1, CIN 2, and CIN 3 were terms used to describe a continuum of disease, representing basaloid changes extending into the lower, middle, and upper thirds of the cervical epithelium with CIN 3 incorporating both CIS and severe dysplasia. Modification of the Bethesda cytologic and histologic grading was proposed by Darragh and colleagues in 2012. CIN 1 and other manifestations of HPV infection are labeled as lowgrade squamous intraepithelial lesions (LSIL) and CIN 3 s a high-grade squamous intraepithelial lesions (HSIL). Since the histologic changes of CIN 2 are heterogenous, including both premalignant and spontaneously regressing lesions, this system encourages use of molecular tests, such as the HPV-related transformation marker p16ink4, to assign mid-grade abnormalities to either the LSIL or HSIL categories. Although the Bethesda System terminology for reporting the results of cervical cytology has been accepted throughout the United States and is used in many other countries, other terminology and definitions are used in Great Britain, Germany, Australia, and other countries, which with different thresholds for colposcopy and treatment creates some difficulty in comparing study results across countries.
The original four- or five-step Papanicolaou cytologic classification, in which “class 1” was normal or benign and “class 5” was suspicious of invasive cancer; the World Health Organization classification of
mild, moderate, or
severe dysplasia and
carcinoma in situ; and the Richart CIN terminal have been replaced in the United States by
the Bethesda system (
Table 50.2). Development of this consensus system was driven by several factors, including intraobserver variation and a lack of reproducibility. Multiple classification systems without common diagnostic criteria left clinicians unsure about appropriate management.
The Bethesda system was introduced in 1988 and subsequently updated and revised in 1991 and 2001. While cytology allows the identification of various infections and other nonneoplastic findings, the core of any report is the identification of epithelial cell abnormalities, either squamous or glandular. The Bethesda system combined HPV cytopathologic effects, often referred to as koilocytosis, with mild dysplasia or CIN 1 into a category called low-grade squamous intraepithelial lesion (LSIL). More significant lesions— including moderate and severe dysplasia and carcinoma in situ, or CIN 2 and 3—were combined into high-grade squamous intraepithelial lesion (HSIL). Pap tests with cellular abnormalities insufficient for definitive diagnosis of LSIL were categorized as atypical squamous cells (ASC). In 2001, these results were further subcategorized as of undetermined significance (ASC-US) or cannot exclude high grade (ASC-H). Glandular abnormalities less than cancer have been classified as atypical glandular cells (AGC), either not otherwise specified (AGC-NOS) or favoring neoplasia, and as adenocarcinoma in situ (AIS).
In the United States, a typical laboratory that processes cervical cytology from an average, generally low-risk population reports epithelial cell abnormalities in about 5% to 6% of patients. Usually, one half to two thirds of these abnormalities are ASC (2% to 4%), whereas 1% to 2% are LSIL and 0.5% to 1.0% are HSIL diagnoses, with about 0.5% glandular cell abnormalities. The ratio of ASC to SIL diagnoses should
be between 2:1 and 3:1 for most cytology laboratories, and roughly half of ASC-US and more than 80% of LSIL should test positive for high-risk HPV. Most cytology specimens are now processed using automated suspensions of exfoliated cells in liquid-based media (liquid-based cytology, LBC) rather than conventional Pap smears. LBC results in fewer unsatisfactory tests and has a greater yield but does not appear to be more sensitive in detecting true premalignant lesions compared to conventional Pap smears. An important advantage of LBC is the possibility of testing for HPV and other biomarkers from the same liquid-based specimen; women with ASC-US cytology who test negative for high-risk HPV types are at low risk for CIN.
The effectiveness of cytology screening has been demonstrated in organized national screening programs. In the United States, demonstration projects in the 1950s showed cytologic screening identified asymptomatic cancers. In Scandinavian countries, the introduction of national screening programs led to a dramatic decline in cervical cancer. However, screening may not be effective in women before ages 25 to 30. Most women who develop cervical cancer in countries with widespread screening availability have not had regular screening.
Despite its effectiveness in cervical cancer prevention programs, limitations to Papanicolaou test screening exist. A single Pap test has a sensitivity of only 50% to 60%. This means that a single test will not detect cervical lesions in many women. However, the slow progression of CIN before the development of an invasive cancer provides the opportunity for multiple screening cytologies over a period of years. So even with limited sensitivity, if three consecutive tests are negative, there is less than a 1% chance that the patient will have a highgrade cervical abnormality. Other screening problems include lesions that do not shed cells, do not transfer from the collecting device, or are not sampled by the clinician. Rarely, the slide preparation or staining is unsatisfactory. Another reason for screening failure is that cytotechnicians and cytopathologists may fail to identify abnormal cells or may misinterpret dysplastic cells as reactive or metaplastic. Glandular lesions are more commonly missed than squamous lesions. Women who are diagnosed with invasive cervical cancer after a reportedly “negative” Papanicolaou test often have abnormal cells on review of their slides that are few in number or obscured by blood or inflammatory changes. The threat of a lawsuit when cancer arises after cytology interpreted as negative may cause cytologists and cytopathologists to “overcall” an equivocal diagnosis and the gynecologist to recommend colposcopy and possibly cervical biopsy or even treatment in a patient with any hint of abnormality. This increases not only the anxiety and morbidity associated with cervical cancer screening but also the costs.
First popularized by Hinselmann in Germany, the colposcope is a magnifying instrument used to examine the cervix. With the colposcope, vascular changes and other epithelial patterns were recognized and correlated with cytologic and histologic changes. These early neoplastic changes were most prominent adjacent to the squamocolumnar junction (SCJ); this area was called the transformation zone, the area of the cervix that had undergone metaplasia from the prepubertal columnar epithelium to mature squamous epithelium. With time, colposcopic patterns associated with the developing stages of dysplasia and early invasive cancer were described. Adoption of colposcopy in North America was limited by cumbersome German terminology and the association of colposcopy with Nazi experimenters, including Hinselmann’s associate Eduard Wirths. However, by the 1970s, colposcopy with directed biopsy had generally replaced cone biopsy as the triage test for women with cytologic abnormalities.
Following the announcement of the 2001 Bethesda System terminology for reporting the results of cervical cytology, the American Society of Colposcopy and Cervical Pathology has sponsored consensus conferences to develop and update management guidelines, expanding recommendations to cover
results of cotesting. The guidelines are based on 5-year risk of CIN 3+ and prescribe similar management for problems with similar risks. They also incorporate cotesting into follow-up to improve sensitivity of surveillance for persistent or recurrent lesions, with longer intervals between follow-up tests to minimize the risk of identifying transient lesions. When cytology, colposcopy, and histology findings are discordant, expert review may be in order. Multiple biopsies minimize the likelihood of missing CIN 3+.
Once the importance of HPV in cervical oncogenesis had been demonstrated, other investigators suggested that a vaccine might prevent infection and perhaps even eliminate the HPVs in already infected individuals. Koutsky and colleagues tested immunization against HPV type 16 in a clinical trial, prospectively randomizing almost 2,400 young women who tested negative for HPV type 16 infection to three doses of a vaccine made from synthetic capsule protein or placebo. This and other trials showed that vaccinated HPV-naïve women develop essentially 100% immunity to targeted HPV types, with substantial reduction in subsequent CIN. Currently approved prophylactic HPV vaccines are targeted against L1 capsid proteins and thus are HPV-type specific. The quadrivalent vaccine targets the high-risk HPV types 16 and 18, as well as low-risk types 6 and 11, while the bivalent vaccine targets only HPV 16 and 18. Minor side effects are common and include injection site pain and fever. Serious side effects are rare, but anaphylaxis and vagal reactions have been reported. Vaccines appear only prophylactic, with little or no effect on established infections. Multivalent and therapeutic vaccines are in development.
Screening for Cervical Neoplasia
The goal of cervical cancer prevention is the identification and destruction of precancer before the development of invasive disease that threatens life, health, and reproductive capacity. This must be balanced against the harms of screening, including stigmatization from the identification of HPV as a sexually transmitted infection, fear of cancer, and the potential for bleeding, local injury, and preterm delivery after treatment when lesions identified through screening are not destined to lead to cancer. For this reason, screening should not begin until age 21, since the risk of cancer in teens is negligible, and although precancers do occur earlier, they are unlikely to progress to invasion prior to detection in subsequent screening rounds. Similarly, cancer late in life is unlikely among women with long negative screening histories, so screening should cease in such women at age 65. The sensitivity of screening appears sufficient to allow 3-year intervals between Pap tests and 5-year intervals between Pap and HPV cotests. These screening recommendations were revised in 2013 (www.ASCCP.org).
A variety of devices have been devised for cervical sampling, including spatulas, brooms, and brushes. The spatula is rotated with gentle pressure twice around the cervix with the longer lobe in the os to enhance sampling. A broom is also rotated against the ectocervix. An endocervical brush is placed in the cervical canal with some bristles visible and rotated 90 to 180 degrees; deeper penetration and more vigorous rotation increase bleeding without improving cell collection. Cells should be transferred to slide or vial according to manufacturer’s instructions; inadequate cell transfer may result in uninterpretable results.
Visual Inspection
Although colposcopy has been used instead of or in addition to cytology for cervical cancer screening in some areas of Europe and South America, it is time-consuming, requires well-trained clinicians, and is expensive. Simple visual inspection of the cervix after application of acetic acid (VIA) or Lugol iodine (VILI) has been studied in resource-poor settings for cervical cancer screening, alone or in combination with HPV testing. Areas of cervical dysplasia turn white after application of acetic acid and turn yellow after application of iodine. Inspection of the cervix using handheld low-power magnifying lenses can identify many lesions. Although these techniques have proved useful and cost-effective in resource poor settings, they are not appropriate for developed countries because of limited sensitivity and specificity. In most settings, VIA and VILI remain investigational.