FIGURE 22-1 Posture of severe spasticity with elbow flexion, forearm pronation, wrist flexion, thumb-in-palm, and mild digital flexion even in this extreme position of wrist flexion.
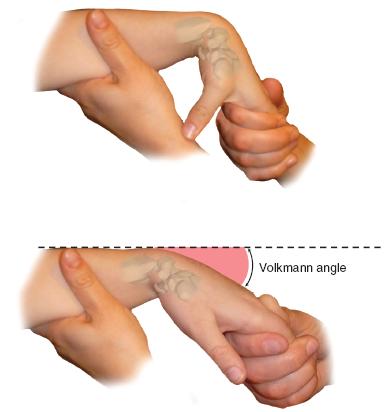
FIGURE 22-2 The wrist is brought from maximum palmar flexion into extension with the digits extended. The angle at which the digits go into a flexed posture is Volkmann angle.
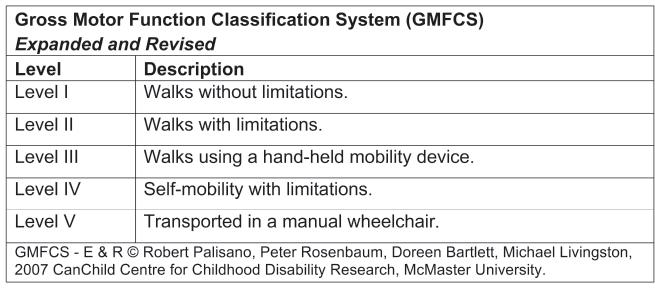
Many classification systems are used for CP patients. The Gross Motor Function Classification System (GMFCS)16 is now commonplace in most CP centers and is used to assess natural history and the effectiveness of therapeutic interventions (Figure 22-3). The Jebsen-Taylor test for hand function, the House classification (Figure 22-4),17 the Melbourne Assessment of Unilateral Upper Limb Function,18 the Assistive Hand Assessment, and the Shriner’s Hospital Upper Extremity Evaluation (SHUEE)19 are among the validated measurement tools for hand and upper limb function in children with C P.20
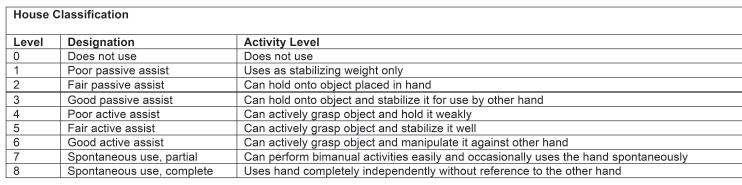
FIGURE 22-4 House classification.
These instruments provide accurate assessment of therapeutic interventions. Of note, the parents’ concept and children’s self-concept of their functional capabilities have been shown to differ greatly by objective assessment.21,22 This implies we need to gather as much information as possible from the child and to use other objective tools. Kinematic 3-D analysis and standardized videotaping of classification testing before and after intervention provide validated scoring and comparisons.23–26 In a disorder as complex as CP hand function, validated instruments are imperative. Dynamic electromyography is used to define in-phase, out-of-phase, and continuous firing activity of agonists and antagonists at specific joints and muscles in certain centers. This can be useful with highly skilled interpretation to determine the best muscles for tendon transfers.
Treatment Indications
Putting is like wisdom…partly a natural gift and partly the accumulation of experience.
—Arnold Palmer
The options for care of the hand and upper limb in CP patients include, among others: (1) natural history27, (2) occupational and physical therapy, (3) splinting, (4) serial casting, (5) electrical stimulation and biofeedback, (6) constraint therapy, (7) botulinum toxin type A (Botox, Allergan Pharmaceuticals, Inc., Irvine, CA) injections, (8) surgery, and (9) a combination of any or all of the above. The goals for each child are to prevent contractures and to maximize grasp, pinch, and release motor functions. Ultimately, interventions are designed to improve functional independence as an adult28,29 and a sense of well-being.
Almost all the children with CP have occupational and physical therapy as a part of their comprehensive care plan. Static splinting is used to prevent contractures due to muscle imbalance and hypertonicity. Most often this is at night or during leisure time so as not to impair functional activities. Daytime static or dynamic splints should truly improve function. These can include wrist extension support, thumb palmar abduction, and swan neck silver ring finger splints if warranted.30
Constraint-induced movement therapy has been shown to improve motor efficiency and quality of movement in some hemiplegic patients.31–33 The constraint methods have included casting, sling use, and restrictive gloves. Repeated constraint therapy has been used effectively.34 However, there are limitations to the levels of evidence on this treatment method.35
Botox injections have been shown to be effective in children with no fixed contractures, good motor control, and a high motivation to rehabilitate.36–38 The most typical injection sites are some combination of the biceps, PT, flexor carpi ulnaris (FCU), finger flexors, and thumb adductor. Standard doses are 1 to 2 U/kg. Both low- and high-dose injections have been shown to be effective. Injections have been performed by palpation and needle electrical stimulation localization. Injections have been administered in the office setting under topical anesthetic cream or local anesthesia or in the operating room under conscious sedation or general anesthesia. Botox injections have been performed by neurologists, developmental pediatricians, physiatrists, and surgeons, among others. Botox is used both as therapy and as a predictor of surgical outcome with reasonable but not unequivocal success at both.39 However, there is a short duration of positive effect and a negative reduction in strength and functional capabilities post injection.40–42 Repeat treatments can be used effectively without adverse effects.43 In our institution, though costly, Botox injections are performed in the day surgery setting with conscious sedation and electrical stimulation localization by our physiatry team of physicians. Postinjection rehabilitation is intended to strengthen antagonists, stretch hypertonic agonists, and learn new functional motor patterns. Repeat injections are only for those who have had a positive effect.
Surgical Indications
The goals of surgery are to improve (1) function, (2) self- or assistive care and hygiene, and (3) aesthetics of the limb. This is done by muscle rebalancing and/or joint stabilization procedures at each affected anatomic level of the upper limb and hand. Although there are not absolute thresholds, ideally the hemiplegic patient should (1) have an IQ > 70; (2) have some discriminatory sensibility or, in its absence, no visual impairment; (3) be between the ages of 5 and 25 years; (4) be highly motivated to perform postoperative rehabilitation; and (5) have no athetosis or flaccidity (see Sidebar ). The quadriplegic patient should have marked contractures that have a negative impact on hygiene, dressing, and mobilization and/or communication. Pain from arthrosis may be a factor in severely contracted wrists in quadriplegia.
SIDEBAR
They say golf is like life but don’t believe them. Golf is more complicated than that.
—Gardner Dickson
There is debate on timing of surgical intervention for hemiplegia upper limb reconstruction. Some surgeons advocate early surgery as it can correct the deformities and rebalance the muscles at a young age. Early surgery (ages 5 to 10 years) has the theoretic and, maybe, real advantage of increased affected side arm and hand use, bimanual skill acquisition, and, therefore, assistive and potentially even independent, hand function. The earlier in life you learn something, the easier it may be to do those activities. These potential advantages have to be balanced against the recurrence with growth and noncompliance risks. Early on in life, the parents are often more concerned than the child about the functional and psychosocial implications of a spastic upper limb and hand. The parents are the drivers of care and compliance with therapy and splints. Later, in adolescence, the young adults who are concerned about their functional level and appearance are motivated to change. This is a huge benefit in terms of compliance with a complex perioperative rehabilitation program that lasts many months. No matter how good you are as a surgeon, you will never perfectly rebalance the muscles, nor will you ever resolve their spasticity with a peripheral operation.
Growth will have a negative impact with ongoing muscle imbalance and spasticity. Unlike the lower extremity and foot where the floor will help maintain correction with each step and upright stance, the hand and arm are free to move without support. The imbalance of neural input and muscle activity can lead to recurrent deformity and loss of function. Second and third operations have less optimal outcomes than primary surgery. Therefore our bias is to do surgery during adolescence in a highly motivated patient whose SHUEE and Jebsen scores indicate a high potential for a positive surgical outcome.
SURGICAL PROCEDURES
The most rewarding things you do in life are the ones that look like they cannot be done.
—Arnold Palmer
Multiple anatomic level surgery is advocated in one anesthetic. Surgery should correct muscle imbalance by (1) lengthening or releasing tight spastic muscles; (2) augmenting weak, stretched muscles by tendon transfers or tenodesis procedures; and (3) stabilizing unstable joints by extra-articular muscle rebalancing, joint capsulodesis, or arthrodesis procedures. In higher functioning patients (House class 3 to 6), tendon transfers and lengthenings are more common, while in lower functioning patients (House 0 to 2), tenodesis and fusion procedures predominate (see Coach’s Corner). We usually perform surgery in adolescents as they are then highly motivated and near the end of growth. This increases compliance, lessens recurrence, and makes sure the surgery is for the affected patient rather than their parent(s) (Table 22.1).
Table 22.1 Surgical treatment options in the spastic upper extremity
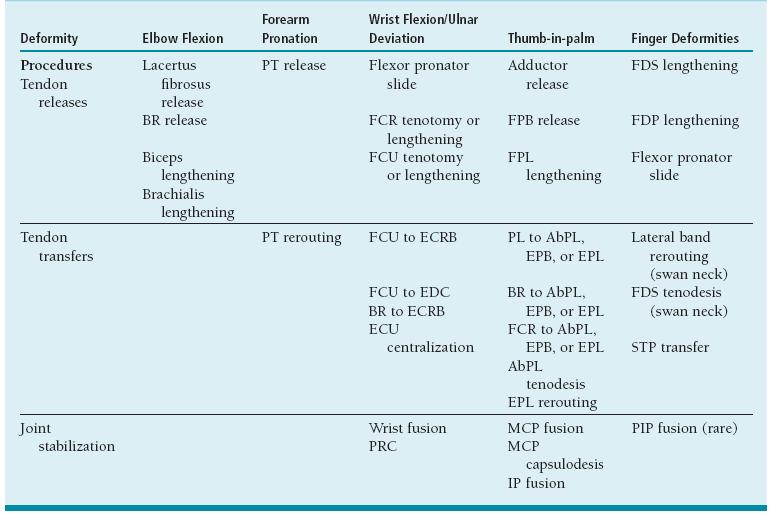
PT, pronator teres; BR, brachioradialis; FDS, flexor digitorum superficialis; FCR, flexor carpi radialis; FPB, flexor pollicis brevis; FDP, flexor digitorum profundus; FCU, flexor carpi ulnaris; FPL, flexor pollicis longus; ECRB, extensor carpi radialis brevis; APL, abductor pollicis longus; EPL, extensor pollicis longus; EDC, extensor digitorum communis; STP, superficialis to profundus; MCP, metacarpophalangeal; PIP, proximal interphalangeal; PRC, proximal row carpectomy; I P, interphalangeal.
From Van Heest AE, House JH, Cariello C. Upper extremity surgical treatment of cerebral palsy. J Hand Surg Am. 1999;24:126
 Hemiplegic Spasticity Reconstruction
Hemiplegic Spasticity Reconstruction
Tendon Transfers, Musculotendinous Lengthenings, Joint Stabilizations
Peter, I can’t follow you hand surgeons with your alphabet soup operations.
—Mike Lynch as a third-year resident
Under general anesthesia and tourniquet control, the initial incision is transverse in the anterior cubital fossa for planned elbow release. Skin and subcutaneous flaps are elevated proximally and distally for more extensile exposure. Venous outflow is maintained. Dissection is carried down to the biceps tendon and radial tuberosity on the lateral side to avoid medial neurovascular injury. The lacertus fibrosus is isolated and released while protecting the underlying median nerve and brachial artery. The musculotendinous portion of the biceps tendon is isolated with a Penrose drain and lengthened. The underlying brachialis muscle is isolated and a musculotendinous lengthening is performed while protecting the antebrachial and radial nerves laterally and the medial neurovascular bundle (NVB) (Figure 22-5). The degree of passive elbow extension is tested and usually comes to near terminal extension. It is rare in hemiplegia, as opposed to quadriplegia, to need to perform an elbow joint anterior release, brachioradialis (BR) release, and/or Z-lengthening of the biceps tendon. The wound is closed quickly in layers with absorbable suture as precious tourniquet time is a-wasting.
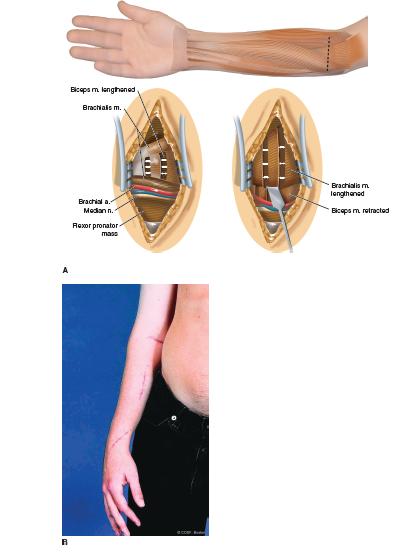
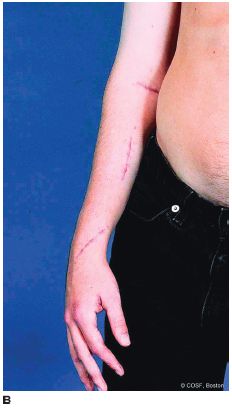
FIGURE 22-5 A: Incision and technique for musculoten-dinous lengthening of biceps and brachialis for an elbow flexion contracture. B: Postoperative elbow extension from release along with FCU transfer, FDS and FDP lengthenings, and thumb release and transfers.
A longitudinal incision is made in the forearm (Figure 22-6A). This is used to isolate the PT proximally and the musculotendinous portions of the flexor digi-torum profundus (FDP), flexor digitorum superficialis (FDS), flexor pollicis longus (FPL), FCU, and flexor carpi radialis (FCR). The PT insertion is identified below the BR while protecting the radial artery and radial sensory nerve. The supinator insertion is proximal, and the FPL origin is distal on the radius. The PT tendinous insertion is elevated sharply off the radius with a long, wide strip of periosteum. This requires sequential use of a sharp knife, cautery, and an elevator to maintain the integrity and length of the tendon as its insertion is naturally well attached to the bone and can be disrupted by careless dissection. A locked whip suture is placed in the tendon and periosteum. Care is taken to be certain there is free excursion of the PT while protecting its proximal neurovascular pedicle. A wide elevation of the interosseous membrane is performe at the level of planned rerouting transfer. With a curved passer, the PT is rerouted under the medial side of the radius and sewn into drill holes on the anterolateral side. The PT will now act as an active supinator.
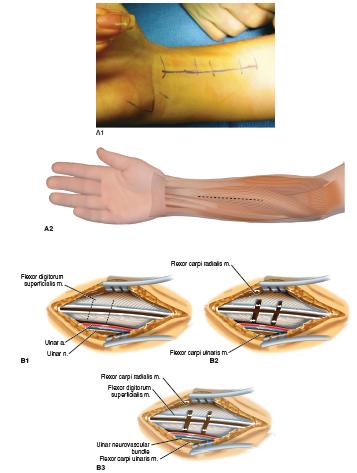
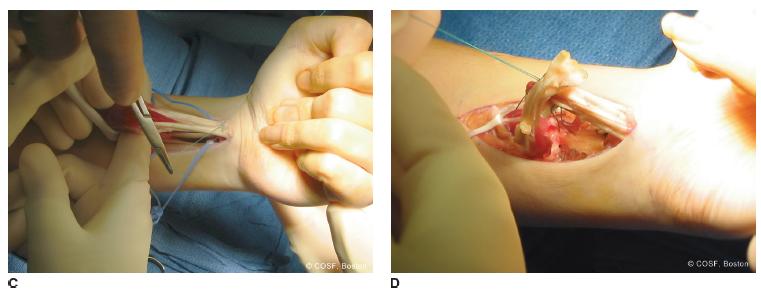
FIGURE 22-6 A1, A2: Straight volar incision used for flexor tendon releases either by musculotendinous lengthenings or STP transfers. B1, B2, B3: Illustration of musculotendinous lengthenings of FDS, FDP, and, if appropriate, FPL and FCR. One or two incisions can be made in each tendon depending on the length needed. C: The FDS tendons have been sutured together as distal as possible. The same is done with the FDP as proximal as possible. D: The FDS to FDP transfer is repaired with the desired cascade. (A1 © COSF Boston.)
In sequence, the FDS and FDP to the index, long, ring, and small fingers are lengthened (Figure 22-6A). Single or dual tenotomies can be performed. Oblique, seindentte incisions are performed until full passive digital extension is possible with the wrist in neutral. This is usually sufficient to correct digital flexion tightness without weakening grip, which can occur with Z-lengthenings and superficialis to profundus (STP) transfers. In a similar fashion, the FPL is lengthened and, if necessary, the FCR. Again, be careful not to overlengthen the wrist flexors in conjunction with FCU to extensor carpi radialis brevis (ECRB) transfer. This will prevent a wrist extension deformity and difficulty with release postoperatively. The palmaris longus (PL) may be released or used for transfer to the thumb as appropriate.
The FCU is isolated while protecting its proximal neurovascular pedicle and the ulnar artery and nerve as they enter Guyon canal distally (Figure 22-7). The FCU is released off its insertion into the pisiform and mobilized proximally for maximal excursion. There are typically multiple aponeurotic connections between the FCU muscle and surrounding fascia, which need to be released to obtain adequate FCU mobility. A locked whip suture is placed in the tendon in preindenttion for transfer to the ECRB.
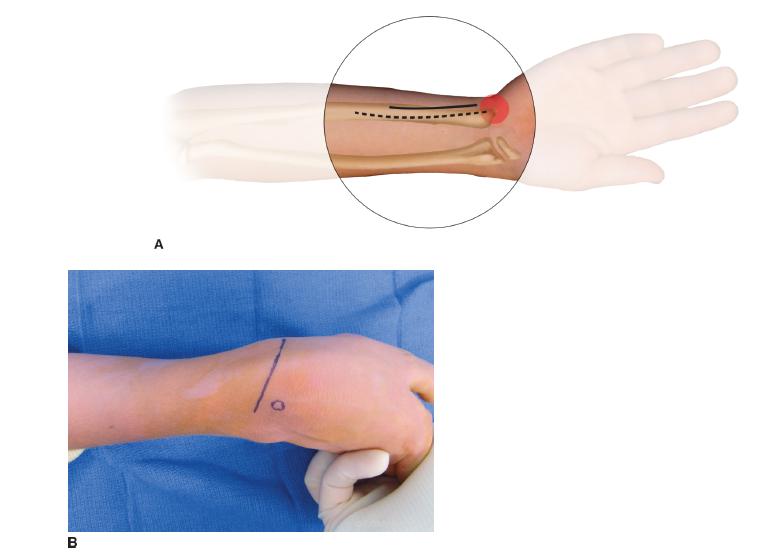
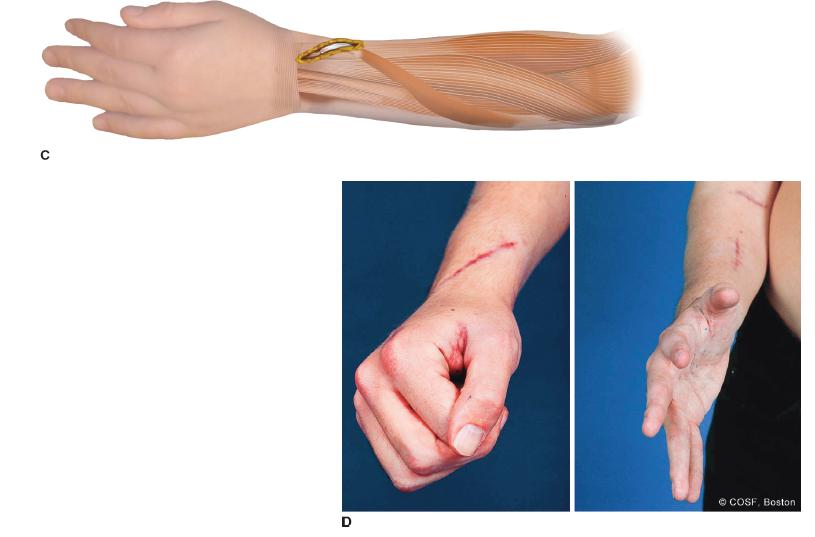
FIGURE 22-7 A,B: Straight ulnar volar incision adjacent to ulnar NVB (outlined by dots) ending proximal to pisiform (outlined by open circle). B: Oblique incision for exposure of EPL and rerouting from 3rd compartment (Lister tubercle noted by circle) to volar to 1st compartment; and ECRB for transfer FCU to ECRB. C: FCU has been mobilized proximally while protecting neurovascular pedicle and is rerouted around ulnar border for weave inset in ECRB. This will improve both wrist extension and supination. D1: Postoperative wrist extension with fingers and thumb flexed (D2 ) and wrist, fingers, and thumb all extended. Oblique incision for FCU to ECRB transfer along with EPL rerouting.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree