Catheter-Associated Infections
Marc Foca
Intravascular catheters are used for a wide range of adjunctive therapies in pediatric patients, such as administering total parenteral nutrition and chemotherapy and facilitating blood drawing. For the purposes of discussing complication risks and preventive strategies, these catheters can be subdivided into short-term, intermediate-term, and long-term devices. Approaches to catheter device placement and care that prevent infection are discussed in Chapters 34 and 107.
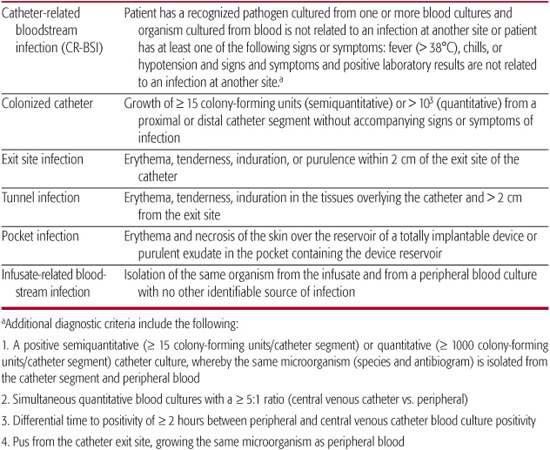
The pathogenesis of bloodstream infections for both short-, intermediate-, and long-term devices includes migration of potential pathogens from the skin at the exit site along the external surface of the catheter to the catheter tip, intraluminal migration of organisms from the catheter hub, contaminated infusates, and rarely, seeding of the catheter hematogenously from a distant focus.1 The true incidence of bowel translocation of microorganisms with subsequent seeding of the catheter is unknown, but this is proposed as a potential mechanism of catheter-related bloodstream infections (CR-BSI) in patients with dysfunctional bowel. Short-term and intermediate-term, noncuffed, nontunneled catheters are more prone to migration of organisms along the external surface of the catheter, and as a result they are infected by a greater proportion of skin flora including coagulase negative staphylococci and Staphylococcus aureus.2-5 Long-term, cuffed, tunneled catheters are less likely to be infected by organisms along the external catheter surface because the cuff acts as a fibrotic dam to migration. The definitions of different types of catheter-related infections are presented in Table 239-1.
Risk factors for catheter-associated bloodstream infections include prolonged use of systemic antimicrobials and the selective pressure for resistant organisms that results, catheter location (short- and intermediate-term lines placed in the femoral vein were more prone to infection in some pediatric studies),6-9 infusion of hyperalimentation with lipids, prolonged duration of catheterization, age < 2 years (especially premature infants with immature skin integrity), burn patients, immunocompromised patients, and those with intestinal integrity issues.1,10
Table 239–1. Definitions of Catheter-Related Infections
 PREVENTION OF CATHETER-RELATED INFECTIONS
PREVENTION OF CATHETER-RELATED INFECTIONS
Maximal barrier precautions during insertion, including the use of sterile masks, gowns, gloves, and large sterile fields, have been shown to significantly reduce the incidence of catheter infections.11-14 Antisepsis with chlorhexadine should be used before insertion of a catheter.15,16 Prior to manipulation of the catheter, hand hygiene and hub antisepsis are mandatory to reduce the incidence of catheter contamination. Sterile gauze, a transparent dressing,17 or a chlorhexa-dine patch18 can be used to cover the site. Preference is usually given to the transparent dressing either coated with or without chlorhexadine because it is easier to evaluate the exit site. Dressings should only be changed when they are loose, soiled, or damp.1 The exit site should be cleansed with chlorhexadine in 70% alcohol, although povidone iodine is still used.19-22 Detailed sample management programs for the care of central venous catheters and peripherally inserted central catheters (PICC lines) are provided on the DVD (Appendix 239-2). To reduce infections, these catheters have been coated with either external chlorhexadine-silver sulfadiazine23 or internal and external minocycline/rifampin.24,25 Both types of catheters have proven effective in reducing the incidence of catheter-related infections in prospective randomized trials in adults when compared to catheters without antibiotic coating, but a recent comparison has found the minocycline/rifampin catheter superior in this respect.24 However, second-generation antiseptic-coated catheters may be more effective in this regard.26-28 A recent meta-analysis found little data indicating that these catheters can be used for durations greater than 2 weeks.29 They have not been extensively studied in children.
The care of long-term central venous catheters is detailed in Chapter 39 and on the DVD (Appendix 239-1). Recent work has shown that vancomycin instilled into the catheter lumen and allowed to dwell for a period of time (antibiotic lock) is effective in reducing the number of Gram-positive infections in these long-term lines.30 The largest prospective study performed in a pediatric population evaluated the use of ciprofloxacin/vancomycin in a similar manner to reduce Gram-negative infections but failed to show any further reduction in infections when compared to vancomycin alone.31
 DIAGNOSIS OF CATHETER-RELATED INFECTION
DIAGNOSIS OF CATHETER-RELATED INFECTION
Our ability to accurately diagnose these infections has evolved over time but remains imperfect. Typically, blood cultures are drawn from patients with intravascular catheters if they develop a fever. In the past nontunneled lines were removed immediately if a blood culture became positive. The line tip was then cultured, and if there were ≥ 15 colonies of the same organism on the catheter tip as in the blood, a line-associated infection was diagnosed.2 This method is accurate, but it requires the removal of the catheter, and with the advent of tunneled and totally implanted catheters, a diagnostic strategy that did not require removal of the catheter was sought. Recently, experimental evidence has revealed that it is possible to differentiate between a line-associated infection and bacteremia from another source by utilizing equal-volume quantitative blood culture methods.32-37 If the infection is truly line related, then the largest burden of organisms would be at the catheter tip. As organisms are shed into the bloodstream, they are filtered through the lungs, and as a result, they have a lower concentration in the peripheral blood. Studies have shown that there should be 5 to 10 times the number of colonies from a blood culture drawn through the catheter than that drawn from a peripheral vein in order to diagnose a line-related infection.38 This method is sensitive and specific, but it is not widely used because of its labor intensiveness and expense. A new method has been proposed. Newer blood culture analyzers are capable of storing time of entry and time of positivity in the computer; therefore, it is possible to generate standard curves with known quantities of specified organisms. In this way an approximate organism burden can be generated once the machine has categorized a bottle as positive.39-44 This method could become the gold standard after further validation because it is less labor intensive and cheaper than the current quantitative methods.
Controversy remains as to the optimal combination of central and of peripheral blood cultures to obtain in pediatric patients. Studies looking at the sensitivity, specificity, and positive (PPV) and negative (NPV) predictive values of central versus peripheral blood cultures have been predominantly performed in adults.45-47 In children, especially infants and toddlers, equal blood volume is not always guaranteed. As a result, because volume of blood has been shown to predict culture positivity,48 inadequate peripheral cultures could lead to a high false-negative rate compared to a central culture, where blood volume is not as important an issue. Consequently, it has become more common in children to draw blood cultures only from the catheter. The optimal combination of central and peripheral blood cultures must take into consideration the age of the child, the type of catheter, the number of catheter lumens and ability to draw from each lumen, the clinical status of the patient, and the type of blood culture vial (pediatric blood culture vials have an optimal liquid medium–to–blood ratio for smaller volumes of blood). Every effort should be made to obtain an adequate peripheral culture before antimicrobials are initiated as per recommendations.
 TREATMENT
TREATMENT
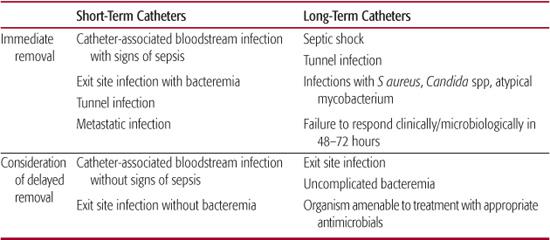
Short- or immediate-term catheters should ideally be removed when a catheter-associated infection is documented.49 Removal of a long-term catheter may not be practical in some clinical situations since this risks the loss of life-saving venous access. It is now routine practice to attempt to treat an infection in a long-term catheter while the catheter is in place. Guidance on decisions for immediate versus delayed removal of a catheter are provided in Table 239-2. A proposed algorithm49 for the management of short- and intermediate-term catheters suggests: (1) removal of catheters when they are no longer needed for patient care and (2) replacement over a guidewire if clinically indicated, ie, mechanical difficulty or suspected but as yet undocumented infection. Replacement by guidewire exchange has been shown to reduce insertional complications. If a line is exchanged because of presumed infection, the old line should be cultured, and if positive, the new line should be removed and another new catheter placed in a different location. If a catheter is removed because of a documented infection, then a new line should be placed in a different location. An alternative approach would be removal of all malfunctioning lines with reinsertion at a different location.
Decisions regarding whether to remove a long-term line secondary to recrudescence of infection is more problematic and should be decided by the pathogenicity of the offending organism, the status of the patient, and the need to maintain appropriate access; however, most experts would recommend line removal in such situations.
The choice of empiric antibiotics depends upon the host, previous history of bloodstream infection, and nosocomial versus community acquired pathogens. There are a large variety of acceptable regimens chosen by the likely organisms and patient status, as shown in eTable 239.1  . In a neutropenic or unstable patient, combination Gram-negative antibiotic coverage should be initiated with agents that work by different mechanisms (ie, a pencillin derivative and aminoglycoside or a quinolone and aminoglycoside) until the patient has stabilized and an organism is identified.
. In a neutropenic or unstable patient, combination Gram-negative antibiotic coverage should be initiated with agents that work by different mechanisms (ie, a pencillin derivative and aminoglycoside or a quinolone and aminoglycoside) until the patient has stabilized and an organism is identified.
Duration of antibiotic therapy depends on the clinical status of the patient and the laboratory findings. If the catheter culture is positive and the peripheral culture is negative, then a short course (3–5 days for coagulase negative staphylococci and 7 days for most other bacterial pathogens) is acceptable as long as the host does not have an underlying immunodeficiency. If the patient is also bacteremic, then the duration of antimicrobials depends on the ability to clear the organism and may prolong therapy by at least a week. Repeat cultures should be drawn before starting therapy if a coagulase negative staphylococcus is isolated because of the possibility of contamination from a skin site; however, this might not always be practical in patients with an underlying immunodeficiency when empiric therapy is initiated quickly. A workup for bacterial dissemination to other sites (spontaneous bacterial endocarditis, bone) be initiated for all patients who fail to clear their peripheral blood culture despite line removal or who remain persistently symptomatic despite negative blood cultures. If this workup is negative at the end of a standard 2-week course of antimicrobial therapy, then it is reasonable to discontinue treatment. In addition, bloodstream infection with Candida (spp) should prompt a search for additional foci of infection, especially in neutropenic patients. It should include an ophthalmology exam, echocardiogram, and CT scan of chest and abdomen (head if appropriate shunting might occur across the heart).
Table 239–2. Immediate vs Delayed Removal of Short-Term and Long-Term Catheters
Adjunctive Treatment Strategies
In addition to the use of antibiotic locks for the prevention of catheter-related bloodstream infections, antibiotic and ethanol locks have been used as an adjunct in the treatment of catheter-related infections. Antibiotic lock therapy has been recommended in the Infectious Diseases Society of America guidelines for the treatment of catheter infections,49 and ethanol lock therapy is a promising new technique for the treatment of such infections.53,54 The major complication of ethanol lock therapy is catheter occlusion, presumably from biofilm destruction. This results in the use of catheter declotting procedures but rarely requires catheter removal (see DVD: Appendix 239-3).
Treatment of Exit/Tunnel Infections
The treatment of more localized exit/tunnel infections depends on the catheter in use.49 For short-term catheters, an exit site infection associated with bacteremia should prompt catheter removal and the introduction of systemic as well as topical antimicrobials. If the exit site infection is not associated with bacteremia, then an attempt can be made to treat the infection with topical agents alone. The treatment of long-term catheter exit site infections is essentially the same, except that unlike the short-term catheter, long-term catheters can be salvaged, even in the face of bacteremia, if the infection responds to appropriate antimicrobial therapy with sterilization of the blood culture and resolution of the local infection. Tunnel tract infections are generally seen with long-term catheters only. They require systemic antimicrobial therapy and usually necessitate catheter removal. Unless bacteremia is present, a trial of empiric therapy for S aureus and Pseudomonas aeruginosa is warranted if the patient is stable. If no improvement is noted, then the catheter should be removed and the tract cultured. Antibiotics can then be tailored to the identified pathogen. Pocket infections require catheter removal for eradication of the offending organism.
 PROGNOSIS
PROGNOSIS
Major complications of central line infections include sepsis, septic emboli, endocarditis, endovascular infection, abscess formation, and death. The true incidence of these complications is unknown in the pediatric population, but prompt removal of the catheter and initiation of long-term antibiotic therapy are required to ensure a good outcome. Certain organisms, including Candida (spp) Pseudomonas (spp) and S aureus, have greater metastatic potential; thus, early consideration should be given to removing central lines when these pathogens are present. 
REFERENCES
See references on DVD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


