CARDIOVASCULAR COMPLICATIONS AND CHRONIC HYPERTENSION
Cardiovascular Complications
Key Points
• Women with heart disease are at risk for cardiovascular complications during pregnancy and have a higher incidence of neonatal complications.
• Knowledge of the hemodynamics of the woman’s condition and the normal cardiovascular/ hemodynamic changes of pregnancy, and how they are likely to interact, is essential to adequate counseling.
Background
Antenatal Cardiovascular Changes
• Blood volume
• Blood volume increases on average by 40% to 60% during gestation, but there is significant individual variation. Blood volume begins to increase early in the first trimester and reaches a maximum in the early third trimester.
• The expansion in plasma volume is greater than the expansion of red cell mass, causing the physiologic anemia of pregnancy.
• With multiple fetuses, the increase in blood volume will be greater still: on average 500 mL more than in a singleton gestation.
• Cardiac output
• Cardiac output increases on average by 40% to 50% during gestation, beginning early in the first trimester and peaking at 20 to 24 weeks.
• In early pregnancy, most of this change results from an increase in stroke volume, whereas later in pregnancy, it also reflects an increase in heart rate.
• Conversely, as pregnancy progresses, supine positioning will decrease cardiac output by 25% to 30% because of compression of the vena cava by the gravid uterus.
• Blood pressure
• Due in part to the smooth muscle relaxing effects of progesterone, systemic vascular resistance decreases during pregnancy, causing a decrease in diastolic blood pressure beginning in the first trimester and becoming maximal in the second trimester. There is also a modest decrease in systolic blood pressure in the second trimester.
• Blood pressure returns to prepregnancy levels in the third trimester.
• Heart size
• With the volume expansion of pregnancy, the heart adapts, as to any chronic volume overload state, by undergoing eccentric hypertrophy. Ventricular chamber size is increased with wall thickness maintained.
• Systolic function (i.e., ejection fraction) is unchanged.
Intrapartum Cardiovascular Changes
• First-stage labor
• With each contraction, there is an increase in circulating blood volume of 300 to 500 mL as blood is expelled from the uteroplacental circulation. This, in turn, increases venous return, maternal cardiac output, and blood pressure.
• The magnitude of the cardiovascular effects of labor is dependent on maternal position and pain management. With the woman in a supine position, the baseline cardiac output between contractions will rise progressively to a maximum increase of 15%. If she remains in a lateral position, the increase will be less (~5%).
• Most of the increase in baseline cardiac output between contractions during labor is caused by maternal pain and anxiety and, therefore, can be minimized by adequate anesthesia.
• Second-stage labor
• With bearing-down efforts to expel the fetus, there will be diminished venous return, causing decreased stroke volume and increased heart rate in an attempt to maintain cardiac output. If these efforts are very frequent, then cardiac output may not return to baseline between contractions.
• Shortening of the second stage by operative vaginal delivery is, therefore, frequently advised for patients with significant cardiac disease.
• Postpartum
• Immediately postpartum, the shunt provided by the uteroplacental circulation is lost. This creates an increase in circulating blood volume and increases in venous return and cardiac output.
• In the days that follow, there is mobilization of extravascular fluid into the vascular system, which further increases circulating blood volume.
• The postpartum period can be a particularly hazardous time for the cardiac patient sensitive to an increase in preload.
Evaluation
Physical Examination
• Cardiac physical examination
• There is an increase in intensity of the first heart sound with exaggerated splitting. A third heart sound is not commonly heard, but the presence of a fourth heart sound should not be attributed to pregnancy.
• Systolic ejection flow murmurs are commonly heard, but diastolic murmurs are rare and should prompt further evaluation.
Diagnostic Evaluation
• Diagnostic evaluation should proceed as necessary during pregnancy. A thorough evaluation of functional status greatly aids counseling regarding maternal prognosis. This evaluation should include a thorough history, physical examination, and diagnostic tests as indicated.
• Functional classification using the New York Heart Association (NYHA) guidelines and the Cardiac Disease in Pregnancy (CARPREG) Investigators (Tables 12-1 and 12-2) has been used to assess the risk of morbidity for the pregnant cardiac patient (1).
• This assessment relies strongly on subjective symptoms; therefore, it is important to substantiate findings with objective testing.
• Chest x-ray films may be obtained safely with abdominal shielding; however, other tests such as echocardiogram probably provide more information.
• Radionuclide perfusion tests such as the thallium scan pose little risk with a fetal exposure of approximately 0.8 rad. Such tests should not be withheld if indicated.
Table 12-1 New York Heart Association Functional Classification of Heart Disease

• Right-sided heart catheterization may be performed for the usual indications without concern for increased risks to the mother or fetus.
• Left-sided heart catheterization should be reserved for cases that clearly cannot be evaluated by other means because the use of fluoroscopy represents a potential risk to the developing fetus.
• There are some differences in physical findings and common studies that may be attributed to pregnancy.
• Chest x-ray. The lordotic posture of pregnancy can create straightening of the left upper cardiac border, mimicking left atrial enlargement. Elevation of the diaphragm by the enlarging uterus causes a more horizontal position of the heart, creating an increase in the cardiac shadow. The pulmonary vasculature frequently appears more prominent.
• Electrocardiogram. The horizontal positioning of the heart due to the elevated diaphragm may result in a left shift of the QRS axis. Minor ST-segment and T-wave changes are commonly seen in lead 3 and aVF lead.
• Echocardiogram. An increase in end-diastolic and end-systolic ventricular measurements is seen with no increase in wall thickness. Ventricular systolic function (i.e., ejection fraction) is unchanged. Mild tricuspid regurgitation is common and is believed to be secondary to increased blood volume.
• Pulmonary artery catheterization. The information regarding hemodynamic measurements by pulmonary artery catheterization in pregnancy is limited to the study of a small number of normal women. Pulmonary capillary wedge pressure, central venous pressure, and mean pulmonary artery pressures appear to be unchanged in pregnancy (2).
Table 12-2 CARPREG Risk Score: Predictors of Maternal Cardiovascular Events
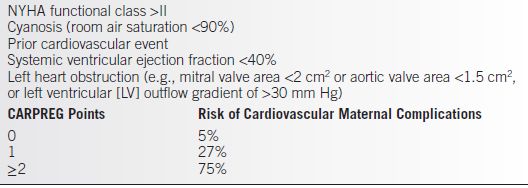
From Siu SC, Sermer M, Colman JM, et al.; Cardiac Disease in Pregnancy (CARPREG) Investigators. Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation. 2001;104:515–521.
Treatment
General Antepartum Management
• Activity level
• During pregnancy, this is individualized to the patient’s level of tolerance as in the nonpregnant cardiac patient. With the increased demands of pregnancy, this may mean restricting activity in cases in which there are significant functional limitations.
• Diet
• It is generally recommended that the pregnant cardiac patient avoid excessive weight gain and excessive salt intake.
• Rapid treatment of stressors
• Additional stressors may lead to rapid decompensation; therefore, it is important to screen for other abnormalities, including anemia, thyroid disease, and asymptomatic infections such as bacteriuria.
• It is important to respond rapidly if abnormalities are found to prevent potential complications.
• Subacute bacterial endocarditis (SBE) prophylaxis
• The rate of bacteremia with vaginal or cesarean delivery is low, and routine SBE prophylaxis is not recommended for most pregnant cardiac patients by the American Heart Association or American Dental Association.
• SBE prophylaxis is recommended at delivery in patients with prosthetic valves, a history of bacterial endocarditis, complex cyanotic congenital heart disease, or surgically constructed systemic pulmonary shunts.
• High-risk procedures that require SBE prophylaxis in pregnant cardiac patients include some types of dental work and some respiratory, gastrointestinal, and genitourinary procedures.
Medications
• Most medications used in cardiac patients may be continued during pregnancy without causing fetal harm. The known exceptions are
• Warfarin (Coumadin), which may cause an embryopathy (nasal hypoplasia, mental retardation, optic atrophy)
• Angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs), which may cause fetal death or intractable neonatal renal failure
• In women with mechanical prosthetic heart valves
• Warfarin appears to be superior to heparin (which has no fetal risks) for preventing thromboembolic complications (2).
• There is some evidence that low molecular weight heparin may be more effective than unfractionated heparin in pregnant women with mechanical valves, but all heparin formulations can result in heparin-induced thrombocytopenia (3).
General Intrapartum Management
• Maternal position.
• Avoidance of supine positioning is critical for mother and fetus.
• In the supine position, there will be obstruction of venous return by the gravid uterus, which will result in decreased cardiac output during contractions and a rebound effect resulting in a higher baseline cardiac output between contractions. The decreased placental perfusion during contractions may result in fetal compromise, and the increased baseline cardiac output may result in maternal compromise.
• Anesthesia should be used liberally because stress and pain in labor will greatly increase cardiac demand.
• Epidural anesthesia is the agent of choice in most cases.
• In cases such as aortic stenosis, in which a decrease in afterload may result in maternal decompensation, slow infusion of epidural anesthetics should be used to minimize peripheral vasodilatation.
• The second stage of labor may be shortened.
• For cardiac patients, maternal exhaustion and frequent Valsalva maneuvers may result in maternal decompensation.
• The choice of forceps or vacuum extraction to expedite delivery should be based on the usual obstetric criteria.
• Cesarean section should be reserved for normal obstetric indications.
• Delivery by cesarean section does not avoid the hemodynamic alterations that occur with vaginal delivery, so trial of labor may be pursued in most cases by following the principles outlined previously.
• Hemodynamic monitoring.
• Use of a pulmonary artery catheter may be advisable in patients with NYHA Class III–IV disease or CARPREG score ≥2 as an aid to avoid major hemodynamic fluxes during labor and delivery.
SPECIFIC CARDIAC LESIONS IN PREGNANCY
Congenital Heart Disease
Background
• There has been a marked increase in the incidence of pregnancies complicated by congenital heart disease because patients who previously would have died in childhood before the availability of modern medical and surgical techniques are surviving to reproductive age.
• Fetal risks include
• Growth restriction due to maternal cyanosis (i.e., tetralogy of Fallot, Eisenmenger syndrome) or decreased uterine blood flow (i.e., coarctation of the aorta, aortic stenosis)
• Inheritance of congenital heart disease (between 5% and 20% risk for most lesions)
Atrial Septal Defect (ASD)
Background
• ASD is the most common heart lesion complicating pregnancy.
• Hemodynamic effects.
• Most patients are asymptomatic, and the lesion is discovered by auscultation of a murmur.
• The left-to-right shunting will cause an increase in pulmonary blood flow, but pulmonary hypertension is a relatively late finding.
• Effect of pregnancy.
• In the absence of pulmonary hypertension, this lesion is well tolerated in pregnancy and should not threaten maternal health. In fact, peripheral vasodilation may reduce left-to-right shunting during pregnancy.
• If the patient has experienced pulmonary hypertension, then she is at risk for Eisenmenger syndrome (see below), a diagnosis that carries a maternal mortality of 50%.
• When counseling the patient with an ASD, it is critical to evaluate her for pulmonary hypertension either by noninvasive testing such as an echocardiogram or by right-sided heart catheterization.
Treatment
• In the absence of pulmonary hypertension, there are no special requirements during pregnancy.
• With pulmonary hypertension, the management should be as for any patient at risk for Eisenmenger syndrome.
• A small percentage of patients with ASD have atrial fibrillation or flutter, which may be managed conservatively with antiarrhythmic agents such as digoxin.
Ventricular Septal Defect (VSD)
Background
• Hemodynamic effects
• The size of the defect frequently determines the degree of disability.
• A small lesion is hemodynamically insignificant and is well tolerated.
• With a large defect with significant left-to-right shunting, the initial abnormality would be left ventricular hypertrophy to compensate for the decrease in forward flow, followed by the development of pulmonary hypertension caused by the increased pressures delivered to the pulmonary vasculature, and, finally, biventricular hypertrophy with the right side pumping against a significant afterload.
• Effect of pregnancy
• The impact of pregnancy will depend on the hemodynamic abnormalities of the individual patient.
• Without pulmonary hypertension, the decrease in systemic vascular resistance caused by pregnancy may be beneficial, causing an increase in forward flow. However, the increase in blood volume may lead to congestive heart failure in the patient with long-standing left ventricular hypertrophy.
• When there is preexisting pulmonary hypertension, Eisenmenger syndrome may develop, a diagnosis that carries a risk of maternal morbidity of 50% (see below).
• The patient with an uncomplicated, repaired VSD is not at risk during pregnancy.
Treatment
• In the absence of pulmonary hypertension, there are no special requirements during pregnancy.
• With pulmonary hypertension, the management should be as for any patient at risk for Eisenmenger syndrome.
Patent Ductus Arteriosus
Background
• Historically, patent ductus arteriosus (PDA) was one of the more common congenital cardiac lesions complicating pregnancy. It is rare today as a result of early detection and surgical repair.
• Hemodynamic effects and consequences are similar to those described for VSD.
• Effect of pregnancy.
• With a small lesion, pregnancy is well tolerated.
• With pulmonary hypertension, there is significant risk for Eisenmenger syndrome, with its attendant risks (see below).
• With a corrected PDA, there is no additional risk as a result of pregnancy.
Treatment
• In the absence of pulmonary hypertension, there are no special requirements during pregnancy.
• With pulmonary hypertension, the management should be as for any patient at risk for Eisenmenger syndrome.
Coarctation of the Aorta
Background
• Coarctation is frequently associated with other cardiovascular abnormalities such as bicuspid aortic valve and congenital berry aneurysm of the circle of Willis.
• Hemodynamic effects.
• Upper extremity hypertension is common secondary to the obstruction to outflow, and there is compensatory left ventricular hypertrophy.
• Visible and palpable collateral arteries in the scapular region and lower extremity hypotension are found commonly.
• Effect of pregnancy.
• With uncomplicated coarctation of the aorta, pregnancy is usually well tolerated.
• A minority of cases will be complicated by congestive heart failure secondary to long-standing left ventricular hypertrophy and dysfunction.
• There may be an increased risk for aortic dissection during pregnancy secondary to the effect of maternal hormones on the blood vessel wall architecture.
Treatment
• Hypertension should be controlled to decrease the risk for aortic dissection and rupture. However, aggressive treatment in women with significant coarctation (i.e., gradient of greater than 20 mm Hg) should be pursued with caution to prevent placental hypoperfusion.
Tetralogy of Fallot
Background
• This is the most common cyanotic lesion complicating pregnancy.
• It is a syndrome of pulmonary stenosis, right ventricular hypertrophy, large VSD, and dextroposition of the aorta that overrides the right ventricle.
• The vast majority of pregnant women will have already had full or partial surgical repair because the disease is otherwise usually fatal in childhood or early adulthood.
• Hemodynamic effects.
• There is right outflow obstruction with resultant right-to-left shunting. This delivers blood with decreased oxygen saturation to the systemic circulation.
• Right ventricular hypertrophy will develop as a result of the right outflow obstruction.
• Effect of pregnancy.
• Patients with a full surgical repair before pregnancy are not at increased risk during pregnancy.
• Pregnancy is poorly tolerated in patients who have not had surgical repair, because the decrease in systemic vascular resistance will cause an increase in right-to-left shunting with further desaturation of blood flowing to the systemic circulation. Patients believed to have an especially poor prognosis in pregnancy are those with
 A baseline hematocrit greater than 60%,
A baseline hematocrit greater than 60%,
 Arterial oxygen saturation less than 80%,
Arterial oxygen saturation less than 80%,
 A history of syncopal episodes, and
A history of syncopal episodes, and
 Significant right ventricular hypertension
Significant right ventricular hypertension
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


