Cardiac Infections
Janet A. Stockheim and Stanford T. Shulman
ENDOCARDITIS
 PATHOGENESIS
PATHOGENESIS
Infective endocarditis (IE) in pediatric patients is often associated with an underlying congenital heart defect. However, structurally normal hearts may also be infected.
Establishment of infective endocarditis results from the interaction of several host and microbial factors. Endocardial surfaces damaged by turbulent blood flow attract platelets and fibrin, leading to the formation of a non-bacterial thrombotic lesion. The endocardium, especially when damaged, appears to be a preferential site of microbial adherence and may have some specificity for binding with certain bacteria.1-3
Following endocardial damage, bacterial access to the bloodstream and adherence to endocardial surfaces are required for the establishment of infective endocarditis. It is now thought that the great majority of infective endocarditis develops as the result of transient bacteremia related to activities of daily life,2 but not all bacteria are capable of initiating this process. The presence of several factors, including bacterial surface polysaccharides, endothelial binding proteins, and agglutinating antibodies that clump bacteria, promote adherence of organisms to damaged endocardial surfaces.
Many of the classic manifestations of infective endocarditis (IE) are immunologically mediated. In IE patients with arthralgias and arthritis, splenomegaly, Roth spots, glomerulonephritis, and thrombocytopenia, circulating immune complex levels are significantly higher than in IE patients without these manifestations.
Bacteremia in patients with infective endocarditis is generally low grade and continuous. As much as 10% of cases of infective endocarditis yield consistently negative blood cultures, most often as a result of prior antibiotic therapy. Fastidious organisms with special growth requirements may be difficult to culture. Bacteria infecting right-sided heart lesions may be filtered by pulmonary phagocytes, significantly reducing the number of bacteria in a peripheral blood sample. In evaluating patients for infective endocarditis, it is preferable to obtain at least three separate blood cultures over a 24- to 48-hour period if the patient is stable. Blood should not be drawn through indwelling vascular catheters because contamination may be misleading. If only one of several blood cultures is positive, infective endocarditis is less likely.
Etiologic Agents
Classically, viridans streptococci have been the most common cause of infective endocarditis, progressing along a subacute course in patients with preexisting cardiac lesions in which fever, fatigue, and immune-complex-mediated clinical manifestations develop slowly over weeks or months. Infective endocarditis caused by Staphylococcus aureus has historically followed an acute course with rapid progression and poor outcome, including death, often in patients with normal hearts. Coagulase-negative staphylococci and S aureus tend to infect prosthetic heart valves within 2 months after implantation and are significant pathogens in neonates who require intensive care and intracardiac central lines. Enterococci (especially E faecalis) are well known and important infective endocarditis pathogens. Gram-negative infections of the heart, although infrequent, are increasing in frequency and are most often associated with intravenous drug use, prosthetic or otherwise abnormal valves, invasive procedures, or nosocomial acquisition.
HACEK is an acronym for a group of small, fastidious Gram-negative coccobacilli—Haemophilus aphrophilus, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella kingae. These organisms that normally inhabit the upper respiratory tract are often associated with infective endocarditis when recovered from the bloodstream. Candidal infective endocarditis occurs occasionally, often associated with indwelling catheters. Aspergillus infective endocarditis has been reported in children following open heart surgery. Streptococcus pneumoniae accounts for a small minority of cases of childhood infective endocarditis in both structurally normal and abnormal hearts. Infections at additional sites (eg, meningitis and pneumonia) often accompany S pneumoniae infective endocarditis and, despite antibiotic therapy, intracardiac complications are frequent.6 Widespread use of the conjugate pneumococcal vaccine has reduced the incidence of invasive infection due to S pneumoniae, including infective endocarditis.
 CLINICAL MANIFESTATIONS
CLINICAL MANIFESTATIONS
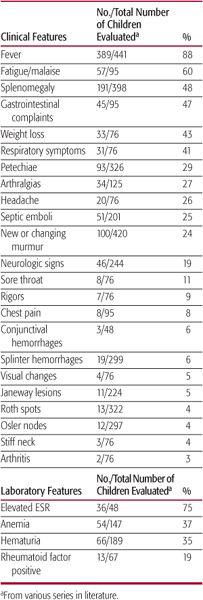
Fever and fatigue are common manifestations of infective endocarditis (IE) in children, whereas other symptoms and physical signs occur with lower frequency (Table 235-1). In a child with underlying congenital heart disease and fever, fatigue, or worsening cardiac function, the diagnosis of IE requires a high index of suspicion. Among all pediatric series, most of the classic peripheral manifestations of IE are infrequent, including Roth spots (small hemorrhagic retinal lesions with pale centers), Osler nodes (small, tender, reddish-purple nodules typically found on the digital pads), Janeway lesions (painless hemorrhagic macules on palms or soles), and splinter hemorrhages (linear streaks in nail beds). Hematuria and associated hypocomplementemia reflecting immune-complex-mediated nephritis occur in less than half of patients, and aseptic meningitis has been reported in a minority of children with infective endocarditis.
Table 235–1. Presenting Features in Children with Infective Endocarditis
 DIAGNOSIS
DIAGNOSIS
Several laboratory tests offer supportive evidence for the diagnosis of infective endocarditis. Continuous bacteremia is typical; multiple blood cultures should be obtained over a 24-to 48-hour period before antibiotic therapy is instituted, if the patient is not highly toxic or septic in appearance. Other common laboratory findings include anemia and elevated erythrocyte sedimentation rate. The presence of rheumatoid factor is dependent on the duration of infection. When present, it offers supportive evidence for the diagnosis of infective endocarditis and is useful for following the patient’s response to therapy.
In 1994, the Duke University Endocarditis Service developed new diagnostic criteria for infective endocarditis based on pathologic evidence or on a combination of clinical findings, and these were modified somewhat in 2000.7 According to the Duke criteria, clinically diagnosed definite infective endocarditis must meet two major criteria, or one major and three minor criteria, or five minor criteria. Major criteria are (1) multiple positive blood cultures for typical infective endocarditis organisms and (2) evidence of endocardial involvement, either echocardiographically or by the development of a new valvular regurgitant murmur. Minor criteria are a predisposition to infective endocarditis (eg, congenital heart defect), fever, vascular phenomena, immuno-logic phenomena, minor echocardiographic findings, and microbiological or serologic evidence that does not meet major criteria. (See also Chapter 490.)
Echocardiography aids in the diagnosis of infective endocarditis.  Transesophageal echo-cardiography may improve the sensitivity of detection of vegetations and is especially useful for visualizing prosthetic valves and the aortic outflow tract. Transthoracic 2D echocardiography has resolution of approximately 2 mm, with sensitivities for diagnosis of infective endocarditis from 59% to 82% in children. Use of transesophageal echocardiography should be considered, when transthoracic views are inadequate, when aortic lesions are suspected, or for optimal evaluation of prosthetic valves.8
Transesophageal echo-cardiography may improve the sensitivity of detection of vegetations and is especially useful for visualizing prosthetic valves and the aortic outflow tract. Transthoracic 2D echocardiography has resolution of approximately 2 mm, with sensitivities for diagnosis of infective endocarditis from 59% to 82% in children. Use of transesophageal echocardiography should be considered, when transthoracic views are inadequate, when aortic lesions are suspected, or for optimal evaluation of prosthetic valves.8
 TREATMENT
TREATMENT
Prior to the availability of antibiotics, infective endocarditis (IE) was uniformly fatal. When patients are clinically stable, at least three blood cultures should be collected over a period of 24 to 48 hours before empiric antibiotic therapy is initiated. In patients with cardiac compromise related to valvular or other cardiac dysfunction or with clinical features highly suggestive of acute IE, collection of multiple blood cultures over a much shorter time period is appropriate, followed by initiation of empiric antibiotic therapy. Following identification of an infecting organism, selection of antibiotics should be based on results of susceptibility testing, eg, penicillin minimal inhibitory concentration for streptococcal isolates.
Some basic considerations apply in determining therapy for infective endocarditis. A prolonged course of therapy with high-dose parenteral bactericidal antibiotics is required. Therapy should be bactericidal rather than bacteriostatic, because the relatively avascular vegetations of infective endocarditis offer little access to host defenses. Guidelines for therapy of specific pathogens, based on susceptibility testing and the presence or absence of prosthetic material, were published by the American Heart Association in 2005.8 Beta-lactams (including penicillins and cephalosporins) and vancomycin are used most frequently. Gentamicin is commonly added for a period of time to achieve synergy with a beta-lactam or vancomycin, especially when S aureus, viridans streptococci, or enterococci are the causative organisms. Rifampin is useful as an adjunct when S aureus infects prosthetic valves. High doses of antibiotics are required to exceed the minimum bactericidal concentration for the infecting organism, and synergistic combinations are recommended in some situations (classically, enterococci) to improve therapy or to shorten its duration. All antibiotic regimens are administered over several weeks, with exact durations dependent on identification and antibiotic susceptibility of the organism, choice of antibiotic or synergistic combination, and the presence or absence of prosthetic material. Generally, regimens are extended approximately 2 weeks in the presence of prosthetic material.
Surgical intervention, in addition to medical therapy, is generally indicated in fungal infective endocarditis or when bacteremia persists despite appropriate antibiotic therapy, when congestive heart failure is uncontrolled by medical therapy, or when abscess of the valve annulus or the myocardium, systemic embolic events, rupture of a valve leaflet or chordae, or acute valvular insufficiency with cardiac failure supervenes. Prosthetic valve endocarditis per se is not an indication for surgery, but early surgical intervention may improve the outcome, especially with staphylococcal infections. Among pediatric infective endocarditis patients, the most frequent indications for surgical intervention are persistent infection despite appropriate medical therapy, embolic events, and worsening congestive heart failure. The aortic valve is most often involved in infective endocarditis that requires surgery, followed by the mitral valve.
 COMPLICATIONS AND OUTCOMES
COMPLICATIONS AND OUTCOMES
The most frequent complications of infective endocarditis are congestive heart failure and arterial embolization. Intracardiac lesions that may lead to congestive heart failure include leaflet dysfunction and valvular insufficiency caused directly by vegetations or by chordal rupture, abscesses of the myocardium or valvular annulus, and myocardial infarction or conduction defects. Pericarditis may result from bacteremic spread of infection or direct extension and usually is associated with S aureus infective endocarditis. Arterial emboli occur most frequently when large mobile vegetations develop on valves, particularly the aortic valve. Although vegetations slowly regress with effective therapy, sudden disappearance of a vegetation should raise the possibility of embolization.
Emboli originating from left-sided vegetations can affect vascular beds in the systemic or cerebral circulation, whereas right-sided lesions produce pulmonary emboli. Mycotic aneurysms most commonly occur at vessel bifurcations and can involve any artery. 
 PREVENTION
PREVENTION
The American Heart Association’s most recent recommendations for prevention of bacterial endocarditis were published in 20072 and are substantially different from previous recommendations. These revisions recognize the lack of evidence to support widespread use of antibiotic prophylaxis and consider its cost effectiveness. Only patients with cardiac conditions with the very highest risk of adverse outcome from infective endocarditis are now recommended to receive prophylaxis for dental procedures (Table 235-2). The mouth appears to be the most common source for organisms responsible for infective endocarditis, and the updated guidelines acknowledge that the risk of transient bacteremia during routine daily activities of oral hygiene greatly outweigh the risk associated with bacteremia related to the occasional dental visit. The importance of good oral hygiene and routine dental care (personal and professional) should be emphasized to all cardiac patients. Table 235-3 provides the AHA’s definition of dental procedures for which prophylactic antibiotics are indicated and their antibiotic recommendations for the limited group of qualified patients. Because normal oral flora may be altered in patients already taking antibiotic prophylaxis (eg, penicillin for rheumatic fever prophylaxis), a different class of drugs (clindamycin, azithromycin, or clarithromycin) may provide more protection against the patient’s own oral microbes in that circumstance.
The genitourinary and gastrointestinal tracts are additional sources of infective endocarditis–causing bacteria, especially enterococci. Prophylactic antibiotic regimens for high-risk genitourinary and gastrointestinal procedures are no longer recommended. However, when patients with cardiac lesions at highest risk of an adverse outcome listed in Table 235-2 have established gastrointestinal or genitourinary infections or receive antibiotics for wound or sepsis prophylaxis during a gastrointestinal or genitourinary procedure, their antibiotic regimen should include enterococcal coverage.
ACUTE RHEUMATIC FEVER
Acute rheumatic fever is a nonsuppurative sequela of pharyngeal infection with group A streptococcus. Target organs of the inflammatory process include the heart, joints, central nervous system, and subcutaneous tissues. Cardiac involvement may be lifelong and is the most important consequence of this disease.
 EPIDEMIOLOGY
EPIDEMIOLOGY
Acute rheumatic fever (ARF) occurs most often in the winter and spring seasons, and most commonly in children ages 5 to 15. Familial susceptibility to ARF may be related to the presence of specific human leukocyte antigens or other genetic markers.9-11 Asian/Pacific Islander children have recently been identified as a group with a possibly increased genetic susceptibility.12 Patients with ARF have a high likelihood of developing it again when rein-fected with group A streptococcus; this tendency declines with age and with increased time since the last episode.
Antigenic differences among group A streptococcus serotypes are related to the bacteria’s M protein, found within its cell wall. Recent data demonstrated a shift in prevalence of many of the “rheumatogenic” M types13-15 to “nonrheumatogenic” M types causing streptococcal pharyngitis in the United States over the past 40 years that parallels the decrease in the incidence of acute rheumatic fever over this period.13
Table 235–2. Cardiac Conditions with the Highest Risk of Adverse Endocarditis for Which Prophylaxis with Dental Procedures Is Recommended



