2Edinburgh Breast Unit, Western General Hospital, Edinburgh, UK
Overview
- The number of women with carcinoma in situ continues to increase and comprises approximately 25% of all ‘malignancy’ detected through screening
- Localised DCIS can be treated by breast-conserving surgery with or without radiotherapy
- The role of hormone therapy in preventing recurrence of DCIS after breast-conserving surgery continues to be investigated
- For patients with larger areas of DCIS, mastectomy with or without breast reconstruction is effective
- Factors that influence local recurrence in DCIS after breast-conserving surgery include completeness of excision, radiotherapy, patient age and histological grade
Carcinoma in situ
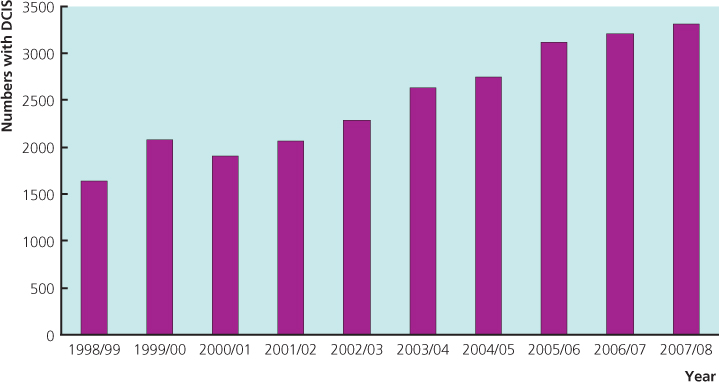
Two main types of non-invasive (in situ) cancer can be recognised from the histological pattern of disease and cell type (Table 16.1). Ductal carcinoma in situ is the most common form of non-invasive carcinoma, making up 3–4% of symptomatic and 20–25% of screen-detected cancers. It has increased in frequency because of the widespread use of screening mammography (Figure 16.1). The increase is across all age groups, with a 12% annual increase in the 30–39-year age group and an 18.1% annual increase in women over the age of 50. Ductal carcinoma in situ is characterised by distortion, distention and complete involvement by a similar and neoplastic population of cells of adjacent ducts and lobular units (Figure 16.2). By contrast, lobular carcinoma in situ, now known as lobular intraepithelial neoplasia (LIN), which incorporates what was previously known as lobular carcinoma in situ (LCIS) and atypical lobular hyperplasia (ALH), is rare (<1% of screen-detected cancers) and presents as relatively uniform expansion of the whole lobule by regular cells with regular, round or oval nuclei. While each involved lobular unit has a uniform cellular population, the pattern and even cytology often do vary between units, with some intervening ones being minimally involved or uninvolved. Despite the ease of separating these two processes most of the time, there are cases with combined features that should be regarded as having clinical features of both processes.
Figure 16.2 Ductal carcinoma in situ: cribiform DCIS (top left); calcification in an area of DCIS (top right); comedo DCIS (bottom left); micropapillary DCIS (bottom right).
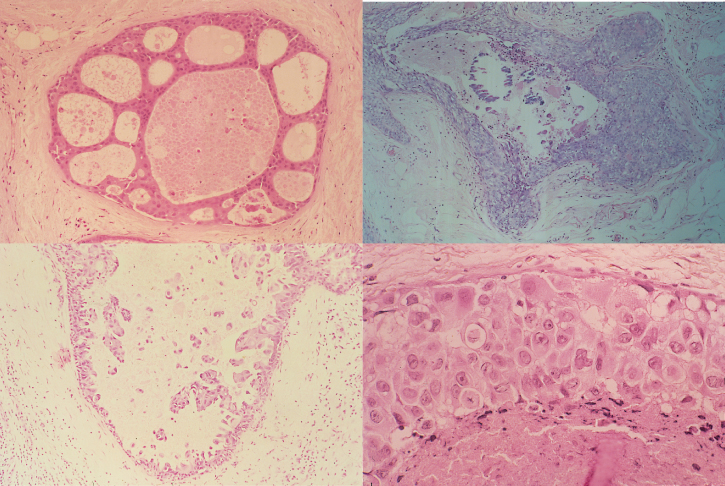
Table 16.1 Features of ductal and lobular carcinoma in situ.
| DCIS | LCIS | |
| Average age | Late 50s | Late 40s |
| Menopausal status | 70% postmenopausal | 70% premenopausal |
| Clinical signs | Breast mass, Paget’s disease, nipple discharge | None |
| Mammographic signs | Microcalcifications | None |
| Risk of subsequent carcinoma | 30–50% at 10–18 years | 25–30% at 15–20 years |
| Site of subsequent invasive carcinoma | ||
| Same breast | 99% | 50–60% |
| Other breast | 1% | 40–50% |
Previously there was agreement about the criteria distinguishing atypical hyperplasia (with specific histological criteria and validation of clinical implications with follow-up studies) from in situ carcinoma. The heterogeneity of some lesions has led pathologists to incorporate LCIS and ALH into LIN. Discussions about classification of so-called DCIS and atypical ductal hyperplasia (ADH) lesions into a single classification of DIN are ongoing. In general, lesions that involve only a few membrane-bound spaces and that measure less than 2–4 mm in their greatest diameter should be regarded as hyperplastic lesions (with or without atypia) and not in situ carcinoma. There is better agreement about larger lesions. Even if there are greatly enlarged lobular units with partial involvement by foci of ADH, this should not be regarded as DCIS for clinical purposes. They are usually in the 5–8-mm size range, and have not been proven to have the natural history of DCIS.
Ductal Carcinoma in situ
Different classifications of ductal carcinoma in situ have been described, and these correlate to some degree with mammographic patterns of microcalcification.
Presentation
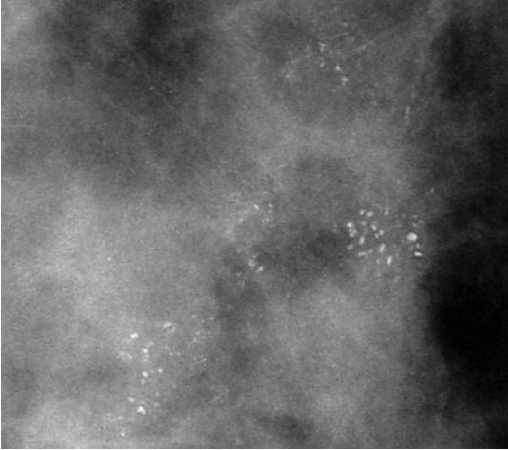
Patients with symptomatic ductal carcinoma in situ present with a breast mass, nipple discharge or Paget’s disease. Screen-detected carcinoma is most commonly associated with microcalcifications (Table 16.2; Figure 16.3), which may be localised or widespread and are characteristically branching within the involved duct system and of variable size and density.
Table 16.2 Classification of DCIS.
Natural Course
Several studies have assessed the risk of subsequent invasive carcinoma in patients in whom ductal carcinoma in situ was not diagnosed by the pathologist or the diagnosis was made but mastectomy was not performed. These studies relate to low-grade carcinoma in situ and show that approximately 40% will develop invasive cancer over a 30-year period, with the majority of these evolving within the first decade. Those who developed invasive cancer did so at the original biopsy site and were in the group where the biopsy was thought not to have removed all the DCIS. Information on the behaviour of inadequately excised intermediate and high-grade DCIS is derived from therapeutic trials documenting local recurrence of DCIS or the development of invasive cancer. This natural history of intermediate and high-grade DCIS is thus continued disease extension and evolution to invasion.
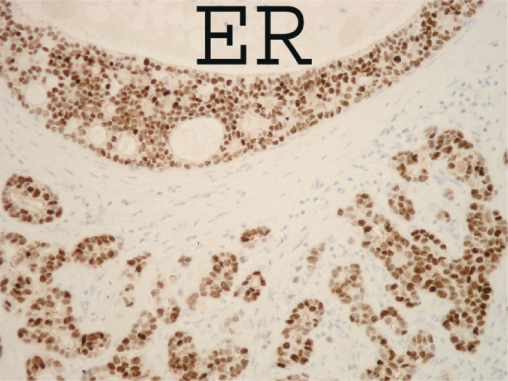
DCIS is a heterogeneous group of lesions, which differ in growth pattern and cytological features, and these different types have marked biological and behavioural differences. Up to 80% of high-grade DCIS overexpress the oncogene or HER2 or erbB2, whereas only 10% of low-grade DCIS express HER2. The presence of a significant amount of oestrogen receptor also differs between histological grades, with 50% (range 16–57%) of high-grade DCIS being oestrogen receptor positive compared with 70% (range 70–91%) of low- and intermediate-grade DCIS (Figure 16.4). Pure cases of micropapillary DCIS, although rare, are often extensive within the breast and frequently involve more than a single quadrant.
Treatment
Symptomatic DCIS usually involves much larger areas of the breast than carcinoma in situ detected by screening and has traditionally been treated by mastectomy (Figure 16.5). Such treatment is associated with excellent long-term outcomes (99% survival at five years). With the advent of breast screening and the use of conservative surgery for invasive carcinoma, wide local excision has been increasingly used for localised carcinoma in situ (Table 16.3). The relative merits of wide excision and mastectomy should be discussed with each individual patient (Figure 16.6). There is an increasing trend to treat DCIS regardless of size and grade by breast-conserving surgery if feasible with or without postoperative radiotherapy.
Table 16.3 Recommended treatment for ductal carcinoma in situ.*
Localised carcinoma in situ (≤4 cm)**/***.
|
*Outside trials of experimental treatments.
**Extent of carcinoma can be estimated in 80% of patients by measuring extent of malignant microcalcification on mammograms.
***Size per se is not an indication for WLE or mastectomy, larger lesions can be treated by WLE in larger breasts.
^Complete excision to clear margins.
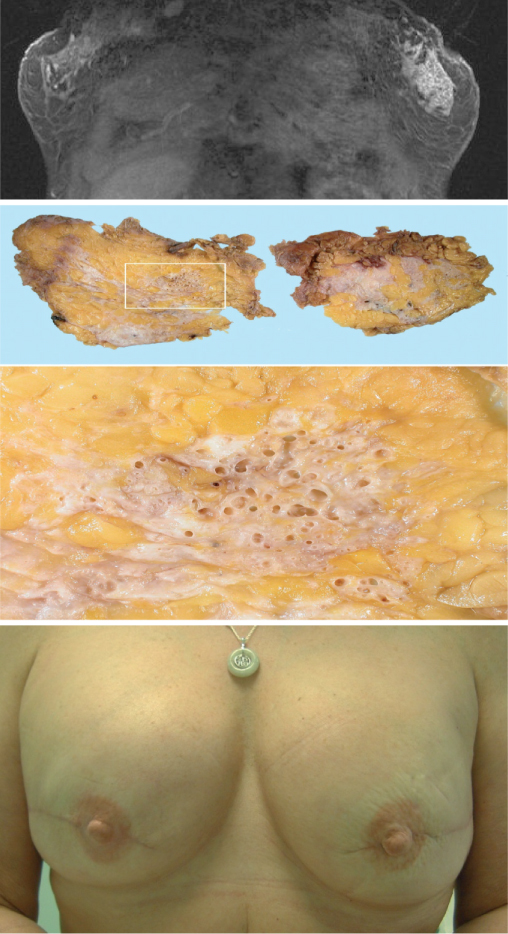
Figure 16.5 Magnetic resonance image (MRI) scan of a patient (top) with a localised area of nodularity in left breast. No abnormality was seen on mammography or ultrasonograpy. Core biopsy showed DCIS and MRI showed a 5 cm area of enhancement that matched the extent of DCIS in the subsequent mastectomy (middle photo). Patient elected to have bilateral mastectomy with immediate reconstruction. Final result after nipple reconstruction and tattooing (bottom).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree