STAGING OF CERVICAL CANCER
In 1937, the Health Organization of the League of Nations adopted a clinical classification system for cervical cancer, making it the first cancer to be classified so. In 1950, this classification was modified to include preinvasive (in situ) cervical cancer, which was designated stage 0. New recommendations for the clinical classification of carcinoma of the cervix were adopted by the General Assembly of the International Federation of Gynecology and Obstetrics (FIGO) in 1961, and several other modifications have been made since. The general use of this classification abroad and in the United States has been extremely helpful in reporting and comparing results of various modalities of therapy. Descriptions of the clinical stages in carcinoma of the cervix uteri as updated by FIGO in 2009 appear in
Table 51.1 and
Figure 51.1.The major redefinition and refinements involve stage I disease. Microinvasive (stage IA) carcinoma has been subdivided into stage IA1 and IA2 based on the depth of cervical stromal invasion by carcinoma. Stage IB has been subdivided into stage IB1 and IB2 based on the size of the clinical lesion.
HISTOPATHOLOGY
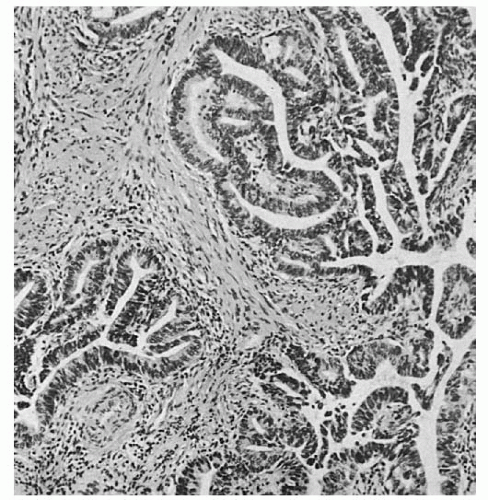
The principal histologic type of invasive cervical cancer, occurring in approximately 80% of cases, is the
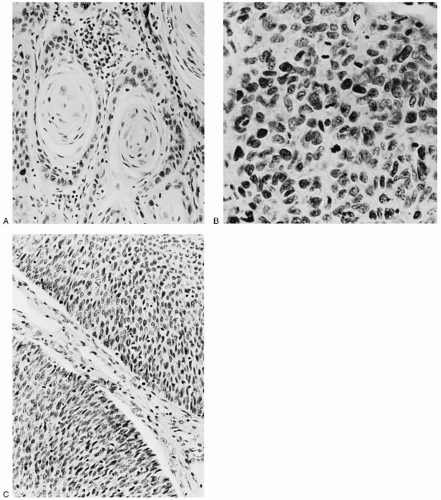
squamous (epidermoid) lesion. In 1923, Martzloff classified these squamous tumors into three main histologic subtypes and grades. Grade 1 tumors contain well-differentiated cells, keratin, and squamous pearls (
Fig. 51.2A). Grade 2 tumors, the most common, are predominantly composed of transitional cells of the large cell nonkeratinizing type (
Fig. 51.2B). Grade 3 tumors, the least common, are poorly differentiated small basal cell-type tumors (
Fig. 51.2C). The classification of Martzloff did not prove to be clinically useful, mainly because biopsies taken from different areas of the same tumor often show different degrees of differentiation and different predominant cell types. Martzloff’s work did inspire Broders, Wentz and Reagan, and others to continue to categorize the histologic types and degree of differentiation of squamous cell cervical tumors and to study their clinical behavior and response to treatment. The histologic classification of squamous cell tumor types introduced in 1959 by Wentz and Reagan is sometimes used in pathology reports. However, Willen and coworkers were unable to confirm a predictive value for survival from the Wentz-Reagan classification. Similarly, most recent studies, including those by the Gynecologic Oncology Group (GOG), have shown that grading squamous carcinomas has little predictive value.
A rare form of squamous cell cancer of the cervix is a verrucous carcinoma. It is a very well-differentiated squamous cell carcinoma with extensive keratinization that usually presents as a large bulky tumor of the cervix and often is confused with giant condylomas, such as those seen on the vulva. There is a sharp line between the tumor and underlying cervical stroma. Verrucous carcinoma, like most cervical cancers, has been shown to be associated with human papillomavirus (HPV) infection. Although metastatic disease is rare, this tumor has been noted to become more virulent if treated with irradiation. Goldberger and coworkers reported an unusually aggressive verrucous carcinoma of the cervix. According to deJesus and coworkers, at least 49 cases of this tumor have been reported in the female genital tract, sometimes as verrucous carcinoma and sometimes as squamous papillary tumor.
Adenocarcinomas of the cervix are becoming more common, especially in younger women. In a review of the Surveillance, Epidemiology, and End Results (SEER) Cancer Incidence Public-Use database from 1973 to 1996, Smith and colleagues reported that although the age-adjusted incidence rates per 100,000 for all invasive cervical cancers and squamous cell cancers decreased by 37% and 42%, respectively, the rates for adenocarcinoma of the cervix increased by 29% during the study period. These results suggest that current screening practices may be insufficient in detecting a significant proportion of adenocarcinoma precursor lesions.
Approximately one half of cervical adenocarcinomas are exophytic, usually polypoid, or papillary; others diffusely enlarge or ulcerate the cervix. Approximately 15% of patients have no visible lesion because the epicenter of the carcinoma is within the endocervical canal. Even without visible signs or symptoms, the lesion may infiltrate deeply into the cervix. Drescher and colleagues reported a higher frequency of uterine corpus invasion and nodal metastasis in 26 patients with cervical adenocarcinoma compared with 139 cases of squamous cell carcinoma. More recent studies have reached contradictory conclusions regarding the prognostic significance of this histology compared to the squamous counterpart. In general, most practitioners would treat stage I cervical cancer of squamous or adeno histology in a similar manner, without much regard to the histology. This is drastically different from the approach to stage I small cell neuroendocrine tumors and other rare tumors, such as adenoma malignum, which tends to be much more lethal.
In addition to pure (endocervical) adenocarcinoma (
Fig. 51.3), cervical adenocarcinomas can exhibit a variety of patterns and can be composed of diverse cell types. Other histologic patterns include
endometrioid, clear cell, serous, mesonephric, and
adenoma malignum. Different histologic patterns and cell types often appear in the same cervical tumor. Because mixtures are common, the designation of tumor type is based on the predominant component. In general, if a second histologic type constitutes 20% or more of the tumor, the lesion is designated as a
mixed cell type, but pathologists also use various criteria to distinguish mixed lesions. Not infrequently, an adenocarcinoma and squamous cell carcinoma coexist in the same tumor, and these lesions are referred to as
adenosquamous carcinomas. The so-called glassy cell adenocarcinoma of the cervix is rare and considered a variant of poorly differentiated adenosquamous carcinoma. It is known to be clinically
aggressive, with frequent early distant metastasis.
Clear cell adenocarcinoma of the cervix can occur in the presence or absence of intrauterine exposure to diethylstilbestrol (DES). Saigo and coworkers found that the endometrioid pattern was associated with a more favorable prognosis than any was other histologic type of cervical adenocarcinoma; however, other authors believe that the subpatterns have no prognostic significance. DES-related neoplasia is rarely seen by most practitioners, but clear cell neoplasia of the cervix unrelated to DES is occasionally encountered.
The early classifications of squamous cell carcinoma of the cervix proposed by Martzloff and others divided these tumors into three categories: keratinizing squamous cell carcinoma, large cell nonkeratinizing squamous cell carcinoma, and small cell carcinoma. Over the years, however, it became apparent that the subgroup designated as small cell carcinoma was composed of a heterogeneous group of tumors, many of which displayed neuroendocrine differentiation. Recent changes in the nomenclature have led to the subdivision of these neuroendocrine tumors into four categories: carcinoid, atypical carcinoid, large cell neuroendocrine carcinoma, and small cell carcinoma. Typical carcinoid and atypical carcinoid tumors are rare in the cervix; therefore, their clinical and pathologic features have not been well characterized. Large cell neuroendocrine and small cell carcinomas are highly aggressive neoplasms, with a propensity to metastasize early and widely. Usual methods of therapy incorporate systemic chemotherapy to treat potential distant metastasis, even in apparent clinical stage I disease.
Various cervical sarcomas have been described by Rotmensch and coworkers. These tumors constitute less than 0.5% of all cervical cancers and include adenosarcomas, leiomyosarcomas, carcinosarcomas, and rhabdomyosarcomas. Melanoma may also develop in the cervix and upper vagina and is often highly lethal. It is extremely rare for a lymphoma to develop primarily in the cervix, but lymphoma in the cervix is more likely to represent evidence of generalized lymphomatous disease.
CLINICAL PRESENTATION
Invasive cervical cancer is more likely than are its intraepithelial precursors to cause symptoms such as abnormal vaginal bleeding (menorrhagia, metrorrhagia, postcoital bleeding, or postmenopausal bleeding) or discharge. Many patients describe a profuse and often malodorous discharge, especially when the disease is advanced. Thus, any patient with abnormal vaginal bleeding or discharge should have a pelvic examination, including a speculum examination with visualization of the cervix. Failure to examine and visualize the cervix in a patient with abnormal vaginal bleeding or discharge could result in failure to diagnose cervical cancer.
Pain is not a common symptom in patients with cervical cancer, unless the disease is locally advanced and has invaded the adjacent pelvic structures, including pelvic nerves. In more advanced stages, patients may report bladder and rectal symptoms. When the disease involves lumbosacral and sciatic nerve roots and the lateral pelvic sidewall, chronic pelvic pain radiating down the leg can be excruciating and indicative of advanced disease. Edema of the lower extremities likewise indicates tumor obstruction of lymphatic and/or venous drainage and is a sign of advanced disease. Palpable supraclavicular or inguinal adenopathy may indicate distant spread and should be evaluated to exclude nodal spread. Ascites is uncommon in cervical cancer. Peritoneal dissemination is uncommon in early stages and is highly lethal regardless of histologic subtype.
Unfortunately, the physician cannot rely on the presence of symptoms to lead to a diagnosis of early carcinoma of the cervix. Many women remain without symptoms for many months. Approximately one third of patients with advanced-stage (III or IV) disease report symptoms for less than 3 months. The only way to diagnose cervical cancer in the earliest possible stages is to routinely apply special diagnostic procedures to large groups of women with and without gynecologic symptoms. This means screening the adult female population with Pap smears and cervical HPV testing at set intervals.
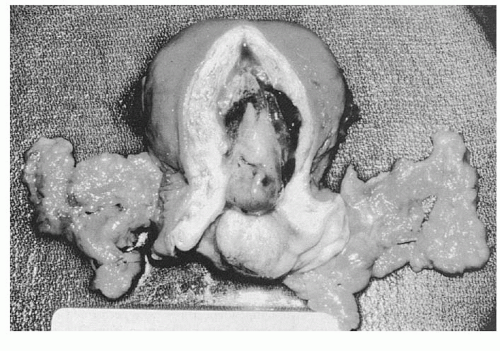
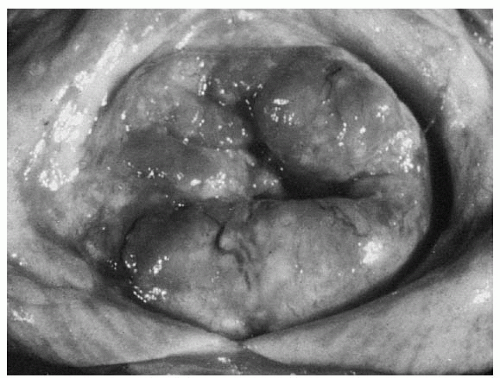
Invasive cervical lesions can be exophytic, infiltrative, ulcerative, or occult. The size of the visible lesion on the cervix may correlate with the depth of stromal invasion, but exceptions do occur with superficial exophytic lesions (
Fig. 51.4).
An everted exophytic carcinomatous growth may be friable. Bits of tissue may break off on the examining fingers. On inspection, the friable exophytic cancer shows a rough, granular bleeding surface that can be sloughing and infected, with a foul-smelling discharge.
A tumor that develops beneath the mucosa of the ectocervix and infiltrates the cervical stroma usually causes cervical enlargement (expansion). The surface of the cervix may still feel smooth, but the cervical consistency to palpation is firm, hard, or nodular. It is characteristic of cervical cancers that develop in the endocervical canal to cause cervical enlargement and a firm cervical consistency before breaking through the mucosa of the exocervix to cause a visible lesion on speculum exam. This also is characteristic of some cervical cancers that develop in postmenopausal women. In fact, it is possible, although uncommon, for a cervical cancer that is developing from an epicenter high in the endocervical canal to invade the parametrial tissue and even obstruct the ureters before causing a visible cervical lesion. An ulcerative lesion can look like a large punched-out ulcer, but more commonly, it is an irregular crater with a necrotic bleeding base and a foul-smelling discharge. The normal contour of the cervix is absent in these ulcerative cases, and complete loss of a significant portion of the ectocervix is common.
Any grossly visible lesion of the cervix should be considered suspicious for cancer, and biopsy should be performed. Good visualization with a speculum and adequate illumination are essential. A Pap smear can be performed, even though it can be less accurate in the presence of a grossly visible cervical lesion, but the main diagnostic procedure should be a biopsy.
Colposcopic examination is neither needed nor particularly effective for a gross cervical lesion but can be helpful when there is a small surface lesion to identify the most abnormal area for directed biopsies. The primary benefit of colposcopy is in visualizing noninvasive, precursor, or minimally invasive lesions that cannot be visualized without magnification.
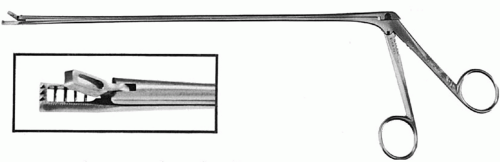
Cervical biopsy techniques are discussed in another chapter. Biopsies can be undertaken with any of a number of special instruments: The Kevorkian (
Fig. 51.5), Younge, or Gaylor biopsy forceps are particularly functional for taking an adequate biopsy specimen. It is important to obtain a sufficient specimen where frank stromal invasion can be demonstrated. Surgical conization under anesthesia is unnecessary when a grossly visible lesion is present. Iodine (Schiller) staining can be used to demarcate the vaginal margins of a neoplastic area from adjacent normal epithelium. All of these procedures, as well as the popular loop diathermy conizations (LEEP), can be done in the outpatient setting. It is rarely necessary to take the patient to the operating room to diagnose cervical cancer. If an anesthetic is deemed necessary for some other reason, a careful pelvic examination under anesthesia, biopsy of any vaginal lesions, cervical and uterine sounding, cystoscopy and proctoscopy (if warranted), and even uterine curettage can be done; therefore, useful information to plan treatment can be obtained.
PROGNOSTIC FACTORS
Several factors have been reported to affect prognosis in cervical cancer. However, the most important determinant of prognosis remains FIGO clinical stage. Based on studies by Piver and colleagues, van Nagell and associates, Delgado and coworkers, and numerous others that have demonstrated the prognostic significance of depth of cervical stromal invasion (DSI) and clinical or histologic tumor size in early-stage disease, FIGO incorporated these factors into its current clinical staging system by subdividing stage I. The 5-year survival rates by stage as reported by FIGO are shown in
Table 51.2.In addition to FIGO stage, other reported prognostic factors include regional (pelvic) and distant (paraaortic) lymph node metastases, lymphovascular space invasion (LVSI), and select histologic subtypes, such as the small cell neuroendocrine tumors mentioned earlier.
Uterine corpus and endometrial cavity extension from primary cervical cancer was originally deemed a poor prognostic factor. The original League of Nations classification of 1937 included such lesions in the stage II category. Over the years, classification of the disease based on extension to the uterine corpus or endometrial cavity has gradually been discounted. These lesions, classified as stage IC in 1950, were included in
the broad category of stage IB in 1961. Despite the change in classification, there is still some concern about patients whose tumor extends into the corpus of the uterus; this is of paramount importance in fertility-sparing radical trachelectomy cases. Evidence from Washington University reported by Perez and coworkers showed that tumor extension into the endometrial cavity lowers the 5-year survival rate of stage IB and IIA lesions by 10% to 20%. Lesions that involve the corpus also were found to have a twofold greater incidence of distant metastases compared with lesions without corpus extension. Similar observations have been reported by Prempree and coworkers from 82 cases of stage I and II disease with endometrial extension. The absolute 5-year cure rates of 68% for stage I and 62% for stage II disease with endometrial extension reflect the higher risk of metastases: 20% for stage I cases and 24% for stage II cases. Such reports must be studied with consideration of the difficulty of establishing a diagnosis of endometrial extension by using microscopic study of endometrial curettage specimens. The curettage specimen is frequently contaminated by the cervical tumor, making it difficult to be certain about endometrial extension. Treatment planning is not altered by such observations, nor is a fractional curettage recommended as part of pretreatment evaluation.
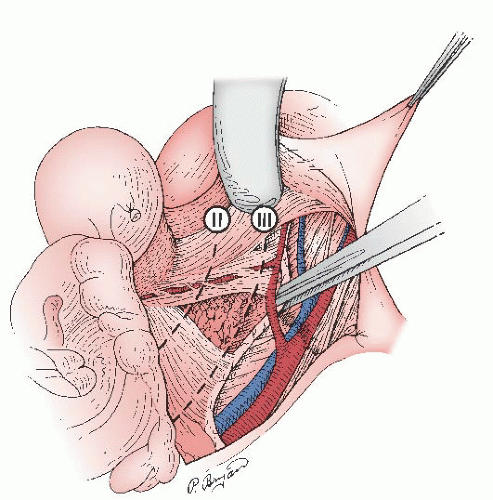
Lymph node metastases, either regional (pelvic) or to higher-level (common iliac and paraaortic) lymph nodes, have proven to be one of the most reliable prognostic factors for patients with cervical cancer (
Fig. 51.6). The frequency of metastases to pelvic lymph nodes is approximately 0% to 1% for patients with stage IA1, 7% to 9% for stage IA2, 12% to 20% for stage IB, 20% to 38% for stage IIA, 16% to 36% for stage IIB, and greater than 35% for stage III and IV disease. Historically, preoperative detection of positive pelvic and paraaortic lymph nodes was unreliable; however, with the advent of newer radiologic imaging techniques, such as positron emission tomography (PET) scan, radiologic imaging has significantly improved the detection of nodal metastasis. Nevertheless, detection of positive lymph nodes is best made by pathologic evaluation and more accurately determined when lymphadenectomy or sentinel lymph node (SLN) mapping is used in preoperative staging or treatment. Although Kolstad showed that when intraoperative lymphography is used, 15% to 25% more patients with stage IB disease are found to have positive regional lymph nodes, this technique is rarely used nowadays and is mostly of historical relevance. The development of cervical injection techniques for SLN mapping has become a more accepted strategy in conjunction with pelvic lymphadenectomy to map early cervical cancer and determine nodal status.
When patients with stage IB cervical cancer are primarily treated with radical hysterectomy and pelvic lymphadenectomy, the 5-year cure rate is approximately 90% if there are no lymph node metastases. However, if metastatic disease to lymph nodes is detected, the 5-year cure rate falls to approximately 65% to 80%. The number of positive nodes also influences prognosis. In a review of the literature before the current standard of postoperative concurrent chemoradiation therapy,
Hoskins reported an 83% survival rate for patients with stage IB and IIA disease who had negative lymph nodes at the time of radical hysterectomy and pelvic lymphadenectomy. The survival rate decreased to 57% in patients with one to two positive nodes and 31% in those with more than three positive nodes.
Metastatic disease to paraaortic lymph nodes occurs in 4% to 7% of patients with stage I disease, 15% to 20% with stage II disease, 25% to 30% with stage III disease, and 30% to 50% with stage IVA disease. Most studies confirm that metastasis to paraaortic nodes occurs more frequently when positive pelvic nodes also are present. The rarity of patients found to have isolated positive paraaortic nodes when pelvic nodes are negative raises the question: How well sampled and/or sectioned were the pelvic nodes? With more incorporation of SLN mapping and pathologic ultrastaging of pelvic nodes, it is likely that the incidence of isolated aortic nodal metastasis will be even less than previously reported. Even with extended-field radiation therapy, the 5-year survival rate for patients with metastases to the paraaortic nodes is only approximately 25% to 35%. Combined modality chemoradiation is a standard of care in many countries for node-positive cases. The addition of more chemotherapy after definitive chemoradiation has been investigated and is gaining popularity as a consolidation strategy in advanced cases.
Historically, tumor grade has been reported to affect prognosis. Early studies by Chung and coworkers and van Nagell and colleagues demonstrated a poorer prognosis among patients with poorly differentiated tumors. However, more recent studies by Zaino and the GOG have shown the grading of squamous tumors to be of little predictive value in cervical carcinoma. Shingleton and Orr reviewed nine publications that reported on 3,761 patients with predominantly squamous cell carcinoma. Twenty-eight different factors were evaluated for prognostic significance. On multivariate analysis, tumor volume, lymph node metastasis, parametrial invasion, and LVSI were found to be significant independent prognostic factors, but patient age and tumor grade were not.
On the other hand, tumor differentiation may have a significant prognostic role in adenocarcinoma of the cervix. Shingleton and Orr also reviewed eight studies of 577 patients with adenocarcinoma of the cervix. As with squamous cell carcinoma, the strongest independent prognostic variables were tumor size and nodal metastasis. However, unlike with squamous cell carcinoma, tumor grade appeared to have prognostic significance.
Several investigators, including Swan and Roddick, Wheeless and Graham, and Julian and coworkers, have drawn attention to the fact that when there is a mixture of adenocarcinomatous and squamous elements—so-called adenosquamous tumors—the prognosis is poor and the incidence of pelvic lymph node metastases is high. Histologic combinations should be considered when comparing the prognoses of adenocarcinoma and squamous cancers of the cervix. The literature is mixed on the overall issue of whether adenocarcinoma in general and adenosquamous cancers specifically are more virulent and less curable than are their squamous counterparts. Stehman and colleagues performed a multivariate analysis of prognostic variables for 626 patients with locally advanced cervical carcinoma treated with radiation therapy on three GOG protocols. Histologic cell type was not found to be a significant prognostic factor. A national pattern of care and evaluation study of the American College of Surgeons also failed to report statistically different 5-year survival rates for squamous and adenocarcinoma, regardless of type of therapy chosen.
In a landmark GOG prospective study of 645 patients with stage IB squamous cell carcinoma of the cervix treated with radical hysterectomy and pelvic lymphadenectomy, Delgado and colleagues identified three independent risk factors in relation to disease-free survival: the depth of invasion, the size of the tumor, and LVSI. The disease-free interval was 89% for patients without LVSI compared with 77% for those with LVSI. Although not all studies have found LVSI to be an independent prognostic factor, as stated, in a review of nine studies (including the study by Delgado et al.) of 3,761 patients, Shingleton and Orr found LVSI to be a significant independent prognostic factor on multivariate analysis. The incidence of LVSI in early-stage lesions varies widely, depending on multiple factors, including the number of sections of the cervix prepared, the depth of stromal invasion, and the interest of the examining pathologist. Observations from Austria and Germany indicate that there is considerable variation in the frequency with which LVSI is recognized by the pathologist. In a combined study of more than 1,000 patients at three different reference centers (Graz, Munich, and Erlangen), Burghardt and associates reported that the frequency with which LVSI was identified ranged from 9% in Munich, where only blood vessel involvement was so classified, to 43% in Graz, where it was classified as capillary-like space involvement. At the third center, Erlangen, the corresponding value was intermediate at 23%. Such variations in histopathologic criteria may well contribute to some of the controversy that exists regarding the prognostic significance of LVSI. LVSI in itself, although a risk factor for nodal metastasis, is not a contraindication to consider and offer fertility-sparing surgery, such as radical trachelectomy.
To this point, this discussion of prognostic factors has included only anatomic and morphologic factors. Peipert and associates emphasized that cancer, including cervical cancer, has both form and function. Accordingly, other clinical variables, such as the patient’s symptoms, symptom severity, and comorbidity, affect the survival rate of patients with invasive cervical cancer. Unless these variables are suitably included such as in nomograms, prognostic estimates based on morphology alone are imprecise, and therapeutic evaluations can be misleading. According to Rutledge and associates, there is no consistent effect of age on survival rate in patients treated for cervical cancer. Younger patients with early-stage disease seemed to survive longer than did older patients, but the tendency reversed when disease was advanced.
PRETREATMENT EVALUATION AND STAGING
When a diagnosis of invasive cervical cancer has been established, the clinician should perform an evaluation of relevant pelvic organs to determine whether the tumor is confined to the cervix or has extended to the adjacent vagina, parametrium, bladder, ureters, or rectum. To determine clinical stage, a pelvic exam with palpation, inspection, and estimation of tumor involvement and measurements is allowed; in addition, a conization is also allowed as a staging tool in occult early-stage lesions. According to FIGO guidelines for clinical staging, diagnostic studies may include intravenous urography (IVU), cystoscopic examination of the bladder and urethra, a proctosigmoidoscopic study, a barium enema (BE), and in the case of early-stage disease, a colposcopic study of the vagina and the vaginal fornices. Colposcopic findings may be used for assigning a stage to the tumor (for instance, FIGO stage IIA), but the results must be confirmed by biopsy. Standard x-rays,
such as a chest x-ray and routine bone films, are allowed for staging. Chest radiographs and electrocardiographic studies are also used to determine cardiopulmonary disease, particularly in the older patient. Advanced imaging modalities with pelvic MRI, computerized tomography (CT), and whole-body PET scan, although very useful to help triage treatment, are not available to the majority of women in developing nations.
When studies detect ureteral obstruction, a tumor is classified as a stage IIIB lesion, regardless of the size of the primary lesion. Ureteral obstruction, either hydronephrosis or nonfunction of the kidney, is well established as an indicator of poor prognosis, as recognized in the FIGO classification system. Retrograde pyelography can be performed after the ureteral obstruction is located for further evaluation, and stenting of the blocked ureter is frequently considered. Kidney function studies such as serum creatinine and creatinine clearance provide important baseline information before treatment; complete urinalysis is useful for detecting the presence of albumin or white and red blood cells and renal tubular casts. The majority of women with advanced cervical cancer will be candidates for chemotherapy, and maintaining good renal function is important in these patients.
In women with bulky or advanced-stage tumors, the bladder mucosa also should be inspected cystoscopically for possible bullous edema, which indicates lymphatic obstruction within the bladder wall. Evidence of tumor in the bladder must be confirmed by biopsy before the lesion can be classified as stage IVA. Urine cytology is usually not sufficient to make that diagnosis. Rectal mucosal lesions from cervical cancer are infrequent and also require a biopsy via proctosigmoidoscopy, because they can be related to an inflammatory or other neoplastic process rather than to the cervical tumor.
A pelvic examination must be performed as part of the staging process, and it may be necessary to have the patient completely relaxed by general anesthesia for a satisfactory exam. In as many as 20% of patients, the initial clinical classification of the disease based on office evaluation has been proven to be incorrect based on findings at the time of pelvic examination under anesthesia. Such an examination can reveal a more advanced stage of the disease than was originally found; additional biopsies (if indicated) or fractional curettage can be done, as well as colposcopy, cystoscopy, and proctosigmoidoscopy. In today’s health care climate, however, the cost of a separate examination under anesthesia may need to be reserved for only the most problematic cases. Moreover, the advancements in pelvic MRI quality are giving gynecologic oncologists better information about the extent of locally advanced cervical neoplasia.
A main goal of tumor staging is to define prognostic groups, to enable clinicians to compare disease status, screening efficacy, treatment results, and other health care outcomes. The present form of the FIGO cervical cancer staging system is in principle a series of definitions of the most important prognostic features of cervical cancer, and it has been used in treatment protocols for generations. However, in the present form of the system, some of the stages do not refer to the presence or absence of paramount prognostic features that are recognized as definition criteria for lower (previous) stages. For example, stage IIA is defined as vaginal vault spread of the disease. Stage IIB does not contain information about the disease involvement of the vaginal vault (presence or absence of criteria for stage IIA), although extensive tumor spread to the vaginal vault seems to be at least as significant a prognostic factor as parametrium involvement (definition of stage IIB). Likewise, stage IIIB does not describe the presence or absence of a condition described in stage IIIA, although extensive tumor spread to the lower parts of the vagina (criteria of stage IIIA) is indeed a severe prognostic sign that should be considered in treatment planning. Similar weaknesses of the staging system include the lack of tumor size description in stage IIB and upper stages. (This problem was recognized by the new staging system, which differentiates between stage IIA1 and IIA2.)
The introduction of a more complex system of stages might better define prognostic groups, but inevitably, it would be more difficult to use and would make it difficult to collect large enough patient cohorts in a given substage for research. However, considering these weaknesses of the FIGO staging, a descriptive combination of the FIGO stages might be used in daily practice. For example, a patient with a bulky tumor involving the vaginal vault, inner half of the parametrium with hydroureter, should be defined as stage IIIB disease (based upon the ureter compression). But the disease (prognosis) might be better defined by adding information about criteria of previous stages, stating, for example, this is stage IIIB disease, with features of stage IIA2 and IIB, that is, no lower vaginal invasion or pelvic sidewall invasion.
Using an extension or combination of findings from lower stages might help to better define prognostic groups, explain results, and create treatment suggestions for special conditions without the need for creating a completely new and more complex staging system.
Pretreatment pedal lymphangiography has been used in the past to detect pelvic and paraaortic lymph node metastases, but the procedure is tedious and associated with many falsenegative and false-positive findings. When compared with lymphadenectomy, positive lymphangiograms have an accuracy rate of less than 75% and a false-negative rate as high as 50%. Furthermore, a lymphangiogram only detects metastatic lesions when the parenchyma of the lymph node has become distorted, by which time the lesions are macroscopic. The procedure is therefore not recommended for routine use in the pretreatment evaluation of cervical cancer patients and is mostly mentioned nowadays for historical value.
Surgical experience from pelvic lymphadenectomy has confirmed an error rate of 15% to 25% in the clinical staging of patients with stage IB or II lesions. In 10% to 30% of cases with stage II or III tumors, in addition to positive findings of occult pelvic lymph nodes, other metastases may be found in the paraaortic nodes. Unfortunately, pelvic examinations and clinical staging as defined by FIGO cannot detect such metastases. Consequently, there is a growing body of literature showing the superiority of cross-sectional imaging (CT, MRI, and PET) over clinical staging in delineating the extent of disease in patients with cervical cancer. As stated earlier, official FIGO guidelines do not incorporate the use of advanced imaging, such as PET and MRI, into the staging of cervical cancer. This is due to FIGO’s determination to create guidelines with universally available staging methods so that staging can be a standardized means of communication between different institutions worldwide. However, as knowledge of prognostic factors and the value of cross-sectional imaging have accumulated, its use in treatment planning has increased despite the lack of change in the official FIGO clinical staging guidelines, and in developed countries, MRI and PET scans are frequently performed as a routine part of the workup and management of suspected advanced cervical cancer.
In a Patterns of Care Study conducted between 1978 and 1988, Montana et al. reported a decrease in the use of IVU (86% to 42%) and BE (58% to 32%) in the staging of cervical cancer patients. During the same time period, the use of CT scan increased from 6% to 70%. In another Patterns of Care Study in the United States recently reported by Amendola
and colleagues, the use of lymphangiography, IVU, and BE had fallen to 1% in the pretreatment evaluation of patients with clinical FIGO stage IB1 or greater cervical cancer who were scheduled for surgery.
The greatest value of a CT scan in the pretreatment evaluation of patients with cervical cancer is in the assessment of advanced disease (stage IB2 and greater) and in the detection and biopsy of suspected lymph node metastasis and possible ureteral obstruction. The treatment plan for patients with locally advanced disease must be modified if retroperitoneal lymph node involvement and/or distant metastasis is discovered. A meta-analysis by Schneidler et al. reported a positive predictive value of 61% for CT scan in the pretreatment evaluation of nodal disease in cervical cancer. Moreover, in experienced hands, fine needle aspiration of retroperitoneal nodes with CT scan guidance has an accuracy rate of 80% to 95%. When the aspiration study unequivocally shows malignant cells, a surgical biopsy need not be performed. This information is most valuable in patients who have metastasis to the paraaortic nodes, because these patients would need the pelvic radiation fields extended to incorporate the involved region if there is no other evidence of distant metastasis. The use of laparoscopy and robotic surgery to “surgically stage” the paraaortic nodes is common in many practices, and pathologic evaluation of these nodes remains the most reliable strategy to exclude disease despite advances with imaging, such as PET scan. Recent studies have shown that even with a negative PET scan in the paraaortic region, a nodal dissection may identify a 10% to 15% nodal positivity on microscopic examination.
SURGICAL TREATMENT OF EARLY-STAGE CERVICAL CANCER
Based on the pretreatment evaluation of the patient, including the prognostic factors of tumor size, clinical stage of the disease, and risk of pelvic node metastases, a treatment schema can be developed for invasive cervical cancer, as shown in
Table 51.3. Almost all patients are treated with either primary surgery or primary radiation therapy with concurrent chemotherapy. Some patients are appropriately treated with combinations of all three. The standard management of patients with early cervical carcinoma is surgical removal of the cervix and pelvic nodal evaluation. The extent of resection of the surrounding tissue depends on the stage, size of the lesion, and the depth of cervical stromal invasion.
Stage IA1 Disease
The exact definition of early-stage cervical cancer has been debated for several decades. This is illustrated by the fact that FIGO changed the definition of early-stage cervical cancer at least five times since 1960. In 2009, FIGO made its most recent revision to the stage IA cervical cancer staging system. After an extensive evaluation of the data in the literature, as well as seeking advice from specialty societies and individuals worldwide, FIGO changed the definition of stage IA1 disease to lesions that invade the cervical stroma ≤3 mm in depth and ≤7 mm in width. Stage IA2 includes patients with >3 mm but <5 mm invasion and ≤7 mm lateral extent.
In an exhaustive review of the literature, Ostor identified 31 studies spanning the years 1976 to 1993 that reported on 3,598 patients with squamous cell carcinoma of the cervix and ≤3 mm stromal invasion. Although not all patients had lymph nodes removed as part of their treatment, the calculated incidence of lymph node metastasis in this group of patients was less than 1%.
Stages IA2, IB1, and Nonbulky (≤4 cm) IIA Disease
The recommended treatment by the NIH Consensus Conference for patients with stage IA2 disease is primary radical or modified radical hysterectomy with bilateral pelvic lymphadenectomy or primary radiation therapy, because the risk of nodal metastases is 4% to 10% in these patients. Again, the diagnosis of both stage IA1 and IA2 diseases should be based
on microscopic examination of removed tissue, preferably a conization or large loop excision specimen, which must include the entire lesion. For stage IA2 disease, the depth of invasion should not be more than 5 mm taken from the base of the epithelium, either surface or glandular, from which it originates. The second dimension, the horizontal spread, must not exceed 7 mm. Vascular space involvement, either venous or lymphatic, should not alter the staging but should be specifically recorded. The remaining stage I cases should be allotted to stage IB. All grossly visible lesions are defined as stage IB.
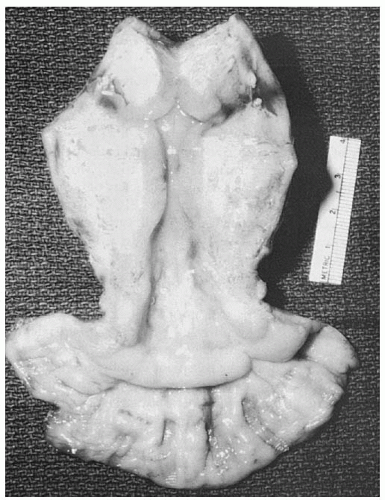
For stage IA2 disease, standard treatment is a type C1 (class III) nerve-sparing radical hysterectomy with bilateral pelvic lymphadenectomy; however, some authors recommend a type B (class II) modified radical hysterectomy (
Fig. 51.8). The type B, or class II, hysterectomy removes the medial half of the cardinal and uterosacral ligaments, ligating the uterine artery at the ureter. This more conservative operation has been used by some authors in the past 3 decades to excise small primary tumors while reducing the partial bladder denervation associated with the complete excision of the cardinal and uterosacral ligaments required for a type C, or class III, hysterectomy. Five-year survival rates of
97% to 98% have been reported for patients with small cervical lesions treated with a type B, class II, hysterectomy. The role of the class II modified radical hysterectomy was recently evaluated in a randomized, prospective study reported by Landoni and colleagues. Two hundred forty-three patients with FIGO stages IB and IIA were randomized to either class II or III hysterectomy. The recurrence-free and overall survivals were similar between the two groups. Patients treated with type II radical hysterectomy had a statistically significant reduction in operative time and postoperative morbidity, particularly bladder dysfunction. However, given the relatively high cure rate for early cervical cancer treated by radical hysterectomy, larger trials are necessary to prove equivalence in survival between the two types of hysterectomy. As stated by Rose, larger trials are required before we can accept these results as the new standard of care. The estimated extent of tissue resection in surgical procedures for early cervical cancer is summarized in
Table 51.4.
RADICAL HYSTERECTOMY
Historical Points in the Development of Radical Surgery for Cervical Cancer
Radical hysterectomy for cervical cancer was performed by John Clark while still a resident trainee at the Johns Hopkins Hospital in Baltimore in 1895; however, earlier reports describe the radical hysterectomy performed in 1888 by the Czech surgeon Pavlik. The procedure is linked in perpetuity to Wertheim of Vienna, who reported his series of 500 cases of radical extended abdominal hysterectomy and partial lymphadenectomy performed from 1898 until 1911. Despite the skill and enthusiasm of Wertheim, Schauta (who developed the vaginal radical hysterectomy in 1901), Okabayashi, and others, radical pelvic surgery was fraught with significant operative morbidity and mortality. Therefore, the introduction of radium brought irradiation to the forefront of primary treatment for carcinoma of the cervix for the next several decades.
In the United States, Joe V. Meigs reintroduced radical hysterectomy as the treatment of choice, publishing a series of 344 cases in 1945. Until formalization of training fellowships in gynecologic oncology in the early 1970s, many outstanding gynecologic surgeons in the United States (Parsons, Ulfelder, Green, and many others) made important contributions and modifications to the radical surgical approach that have markedly decreased complications while preserving the cure rate. Proficient performance of the radical hysterectomy remains a benchmark of the gynecologic oncology surgeon.
Patient Selection for Radical Hysterectomy
Simple hysterectomy is not adequate treatment for stage IB cervical cancer. In 1943, Jones and Jones reported a 5-year survival rate of only 41.6% in patients who had been treated for stage I cervical cancer with simple hysterectomy only. Such poor results also have been reported by Schmidt and others. When more than FIGO stage IA1 invasive cervical cancer is a surprise finding in a simple hysterectomy specimen, additional therapy—usually radiation therapy with or without chemotherapy—should be given postoperatively. Suggested indications for radical abdominal hysterectomy are summarized in
Table 51.5.Although radical hysterectomy and pelvic lymphadenectomy occasionally are used to treat patients with adenocarcinoma of the endometrium with involvement of the cervical stroma (stage IIB) and, rarely, patients who have a small cervical cancer that persists or recurs in the cervix after primary radiation therapy, in this chapter, emphasis is given to the use of the operation as primary treatment for invasive cervical cancer.
In most institutions, the majority of patients with stages IA2, IB1, and nonbulky IIA cervical cancer are offered radical abdominal hysterectomy and bilateral pelvic lymphadenectomy as primary treatment.
Patients with bulky stage IB disease (currently FIGO stage IB2) have traditionally been treated with radical hysterectomy and bilateral pelvic lymphadenectomy or primary radiation therapy, with equivalent survivals. However, patients with these larger lesions treated surgically have a very high risk of having lymph node metastasis or close resection margins or local tumors risk factors, such as deep stromal invasion with LVSI, which are often reasons for postoperative pelvic radiation treatment. Landoni and colleagues performed a randomized trial of radical hysterectomy and pelvic lymphadenectomy versus pelvic radiation therapy for stage IB to IIA cervical cancer. Patients randomized to the surgery arm who had pathologic risk factors, such as lymph node metastasis, received adjuvant radiation therapy. Of the 55 patients with tumors greater than 4 cm, 46 (84%) required postoperative irradiation. The disease-free and overall survival rates for these patients treated with surgery and radiation therapy were the same as those for patients with bulky tumors treated with radiation therapy alone; however, the combination therapy significantly increased morbidity. Subsequently, a randomized trial performed by the GOG has demonstrated the benefit of the addition of cisplatin chemotherapy to pelvic radiation followed by extrafascial hysterectomy in this group of patients. Therefore, many experts feel that patients with FIGO stage IB2 and bulky IIA cervical cancer are best treated with concomitant cisplatin chemotherapy and radiation therapy with or without completion extrafascial hysterectomy.
In the United States, patients with stage IIB invasive cervical cancer usually are excluded from primary treatment with surgery. They are usually treated with concomitant radiation therapy and chemotherapy. However, it should be noted that select stage IIB cases have traditionally been considered in some countries (Japan, Germany, Hungary, and other European countries) as an indication for primary radical hysterectomy. Recent studies from Japan demonstrated equal overall survival with a favorable quality-of-life outcome of radical hysterectomy compared to primary chemoradiotherapy in this stage of the disease. The question about radical hysterectomy indication in select stage IIB patients cannot be considered resolved; thus, although radical hysterectomy treatment of stage IIB cervical cancer is not suggested in this book, it should be considered as an acceptable alternative concept in the appropriate hands and clinical setup.
The clinical significance of parametrial involvement dates to the early studies of Kundrat and Sampson. Kundrat, working in Wertheim’s clinic, studied more than 21,000 serial microscopic sections of the parametrium, finding that the parametrium of one or both sides was involved in 44 of 80 patients. In a similar study at the Johns Hopkins Hospital, Sampson pointed out that the parametrium could feel indurated and yet show no evidence of cancer. Also, the parametrium can feel normal and yet contain cancer. Sampson emphasized that only
by the microscope can the surgeon exclude cancer involvement of the parametrium. More recently, Inoue and Okumura found parametrial extension in 7% of stage IB patients and in only 34% of stage IIB patients. Burghardt and Pickel found true parametrial involvement in only 19% of stage IIB patients. Matsuyama and coworkers found no parametrial cancer in 58% of stage IIB patients. These studies were based on careful examination of microscopic sections and reemphasize the difficulty of being certain about parametrial extension from pelvic examination alone, emphasizing the shortcomings of a clinical staging system. Inoue and Okumura studied 628 operative specimens from patients treated with radical hysterectomy and lymphadenectomy and found that parametrial extension is an important factor in the number of positive lymph nodes found and in patient survival. If there is suspicion of spread into the parametrial tissues by examination, CT scan, or MRI scan, it is reasonable to offer radiation therapy with concomitant chemotherapy as primary treatment (despite the fact that the official FIGO stage should remain unchanged).
The major point to be emphasized is that the gynecologic surgeon should not attempt to treat a patient with a large cervical tumor with primary radical surgery unless there is reasonable assurance that the operation will result in the complete removal of the central tumor with an adequate margin of tumor-free tissue around it. The surgeon should not operate on patients with the idea that radiation therapy with or without chemotherapy can be used postoperatively to eliminate residual fragments of tumor tissue after incomplete resection. Such patients usually are better treated with concurrent pelvic radiation and chemotherapy from the beginning.
Primary radical surgical treatment is not contraindicated in any histologic type of cervical cancer. Shingleton and coworkers have suggested that the survival of patients with stage I adenocarcinoma of the cervix is better with surgery than with irradiation. Patients with stage I adenosquamous cancer, clear cell cancer, and undifferentiated adenocarcinoma have a poorer prognosis, regardless of the method of treatment chosen, and therefore are often considered for adjuvant radiation therapy and/or chemotherapy after primary surgery.
Patients considered for radical hysterectomy must be acceptable candidates for an operation and free of serious medical problems that contraindicate extensive surgery. In former years, some institutions limited radical surgery as primary treatment to premenopausal women so that ovarian function might be conserved. As experience has accumulated, it has become apparent that the operation is also well tolerated by older women. In a study of 45 women aged 65 years and older with cervical cancer, Fuchtner and associates concluded that age alone should not be a contraindication to extensive hysterectomy in the elderly patient with American Society of Anesthesiologists physical status I to III. Kinney and coworkers reported their experience with the Wertheim operation in a geriatric population. Thirty-eight selected women between 65 and 89 years of age (median age, 69 years) were compared with 320 patients younger than age 65. The survival rates were almost identical in the two groups. Perioperative morbidity was minimally increased in the geriatric group. Geisler recently compared the outcomes after radical hysterectomy and pelvic lymphadenectomy of 62 patients older than age 65 to 124 matched controls age 50 or younger. Even using this relatively younger cohort for comparison, there were no significant differences in operative mortality or morbidity. However, to achieve such excellent results in older women, Kinney and coworkers pointed out that “meticulous surgical technique, high-quality ancillary services, and support from internal medicine and anesthesia services” are required. Such extended supportive care is not available in every hospital.
Extreme morbid obesity presents especially difficult technical problems when radical surgery is chosen for primary treatment. Not only is the performance of the operation more challenging and possibly less satisfactory, but also there may be an increased risk of wound complications, postoperative infection, intraoperative hemorrhage, pulmonary embolus, pulmonary atelectasis, and anesthesia-related and other problems. Unfortunately, primary treatment with radiation therapy with or without chemotherapy also is frequently less than satisfactory in extremely morbid obese patients. The introduction of robotic minimally invasive surgery may play a role in treating select obese women with early cervical cancer, avoiding the need for laparotomy.
Studies by Soisson and Levrant compared outcomes after radical hysterectomy for cervical cancer in obese versus nonobese women. They found that survival was not compromised, and the incidence of serious complications was not increased in obese patients. However, in obese women, the authors reported that the operative technique is more difficult, the procedure lasts longer, and the surgery is associated with greater blood loss. Cohn and colleagues recently reported their results with radical hysterectomy for cervical cancer in 46 obese women. The median body mass index was 36 kg/m2, and the median weight was 95 kg. Nine patients (20%) experienced postoperative morbidity, mostly related to wound complications. No patient developed a fistula. Massi and associates have reported that the Schauta-Amreich vaginal hysterectomy can be used as an alternative to the radical abdominal hysterectomy in the presence of obesity or elevated surgical risks.
According to Shingleton, primary treatment with radical surgery paradoxically also can be riskier in very thin patients because of a higher incidence of fistula. It is speculated that easy exposure and lack of excess fatty tissue in these patients may result in removing essential vasculature around the ureters and bladder, resulting in ischemic necrosis. A thin patient has less fat around the pelvic vessels and in the lymph fields; thus, the surgeon should be satisfied to remove less tissue in an operation that will still be adequate in a thin patient.
The management of the
pregnant patient diagnosed with invasive cervical carcinoma is more challenging. First, a decision must be made to either save the pregnancy or treat the cancer. Pregnancy is not a contraindication to primary treatment of stage IB or IIA carcinoma of the cervix with radical surgery (
Fig. 51.9). In 1974, Sall and coworkers reported on 29 patients with stage IB carcinoma of the cervix in pregnancy treated with radical hysterectomy and pelvic lymphadenectomy. At the time of publication, 28 patients were alive and well, and 23 patients had been followed for more than 5 years. There were no fistulae or major complications. Others have confirmed 5-year survival rates of 85% to 95% for patients treated with radical surgery for stage IB cervical cancer in pregnancy. Funnell and associates, operating on 17 pregnant patients, suggested that the associated pregnancy changes facilitated the surgical dissection. Sood and colleagues performed a case-control study comparing the outcomes after radical hysterectomy and pelvic lymphadenectomy for 26 pregnant versus 26 nonpregnant patients. Operative times and postoperative complication rates were similar between the two groups. There was a statistically significant increase in blood loss at the time of surgery for pregnant patients; however, there was no difference in the frequency of blood transfusion. Eleven patients underwent surgical treatment in the third trimester of pregnancy, with a mean planned delay in therapy of 16 weeks. None of the patients with the planned delay in therapy developed recurrent disease. The authors concluded that surgical management of early cervical cancer is safe during pregnancy. In select obstetric patients who desire to maintain
their pregnancies, Sood and colleagues agreed with the conclusions of Greer and coworkers that planned delay in therapy to increase the likelihood of fetal maturity is safe for early-stage I squamous cell cancers diagnosed in the late second and early third trimester. All other patients should be treated promptly in an attempt to cure the cancer.
Finally, based on data from Orr, Chapman, and others, extensive parametrectomy and pelvic lymphadenectomy appear to be appropriate management for selected patients found to have unexpected invasive cancer of the cervix on pathologic examination of a uterus removed for benign conditions. Low morbidity rates and acceptable rates of long-term disease-free survival have been reported. However, pelvic irradiation is usually recommended for these patients.
Advantages of Radical Surgery as Primary Treatment for Invasive Cervical Cancer
The most important considerations in choosing a method of therapy for any cancer are, first, effectiveness of the treatment in curing the disease and, second, mortality and morbidity rates associated with the treatment plan. For the indications listed previously, the cure rates of primary radiation therapy and primary extensive surgery are about equal. The modern mortality rates also are about equal. Both modalities of therapy have a list of complications unique to each that seem about equal. There are, however, important major and minor advantages of primary radical surgery over irradiation for early-stage disease, some of which are discussed in the following sections.
Accurate Evaluation of Extent of Disease
The findings at operation and from careful pathologic examination of the surgical specimen can be immensely helpful in selecting high-risk patients for adjuvant postoperative radiation therapy, chemotherapy, or both. Most patients with FIGO stages IA2, IB1, and nonbulky IIA disease are not found to have high-risk factors and thus are spared the potential morbidity associated with whole pelvic radiation therapy. Furthermore, the findings at operation and careful pathologic examination of the surgical specimen can be helpful in determining prognosis and in identifying patients at greatest risk for persistence or recurrence of disease. Such high-risk patients may require additional therapy.
In addition to an accurate assessment of the extent of the cervical cancer, primary surgical treatment allows for discovery of other intra-abdominal incidental conditions and diseases entirely unrelated to the cancer. Ovarian malignancies, pelvic tuberculosis, sigmoid diverticulitis, cholelithiasis, and other diseases and conditions may be encountered at the time of operation.
Preservation of Ovarian Function
When primary radiation therapy with or without chemotherapy is used to treat invasive cervical cancer in premenopausal women, premature loss of ovarian function is an unfortunate and inevitable result. When primary surgery is used instead, the function of normal ovaries can be conserved. Sutton and associates analyzed the incidence of ovarian metastasis for 991 patients with stage IB carcinoma of the cervix treated with radical hysterectomy and pelvic lymphadenectomy on a prospective GOG protocol. Ovarian spread was found in 4 of 770 patients (0.5%) with squamous cell carcinoma and in 2 of 121 patients (1.7%) with adenocarcinoma. The difference was not statistically significant. All six patients with ovarian metastases had other evidence of extracervical disease. This study confirmed that ovarian metastasis is rare in patients with stage IB cervical cancer and extremely rare in the absence of other evidence of extracervical disease.
Although the incidence of ovarian metastasis is slightly higher in women with adenocarcinoma of the cervix as compared with squamous cell carcinoma, ovarian conservation should still be considered, especially in young women. Brand and Berek reported no ovarian metastases in more than 60 patients with adenocarcinoma of the cervix treated with radical surgery. Angel and associates found no ovarian metastases in 41 patients with adenocarcinoma of the cervix who underwent oophorectomy. Greer and coworkers treated 55 patients with stage IB adenocarcinoma of the cervix with radical hysterectomy and pelvic lymphadenectomy. Ninety-one percent had ovarian preservation, and there was no evidence that this contributed to tumor recurrence. Hopkins and coworkers found that the best cumulative 5-year survival rate (93%) with cervical adenocarcinoma was in patients treated by radical hysterectomy without bilateral salpingo-oophorectomy and concluded that “ovarian conservation seems to be an acceptable alternative to bilateral salpingo-oophorectomy” in young patients.
Some authors have advocated transposing the ovaries into the paracolic gutters at the time of radical hysterectomy in premenopausal women to protect the ovaries from radiation damage should postoperative pelvic radiation therapy be needed. The technique used for ovarian transposition was described by Husseinzadeh and coworkers. However, in the series reported by Chambers and coworkers, there was a threefold increase in symptomatic benign ovarian cyst formation with lateral ovarian transposition compared with those who did not have their ovaries transposed. Furthermore, Anderson and coworkers reported that only 4 of 24 patients (17%) who had ovarian transposition retained ovarian function after postoperative radiation therapy. Moreover, after transposition, 17.6% of patients required surgical treatment for ovarian-associated pain or cysts. These data raise the question of whether paracolic gutter transposition actually achieves its goal of protecting the ovaries.
Sexual Function
Studies of the effect of surgical and radiation treatment for cervical carcinoma on sexual function have been published by Seibel and coworkers from Emory University and by others. Among patients treated with radiation, there are decreases in sexual enjoyment, ability to attain orgasm, frequency of intercourse, and desire for intercourse. Marked alterations can be seen and felt in the upper vagina and paravaginal tissues. The vagina usually is shorter from stenosis. The upper vagina is less pliable. Tissues are fixed and firm. The vaginal mucosa is thin, smooth, and dry, with a tendency to split and bleed with slight trauma. Some of these changes are made more pronounced in young women because of hypoestrogenism from radiation-induced premature menopause. They are not completely reversed by intravaginal or oral administration of estrogen. Such functional and anatomic changes are not seen nearly as frequently in patients treated with primary extensive surgery. Even if the vagina has been surgically shortened by several centimeters with primary surgery, it remains soft, pliable, moist, and functional. Unfortunately, in women who undergo postoperative adjuvant pelvic irradiation, some of these characteristics are lost.
Fewer Late Complications
Late complications after treatment for cervical cancer are rarely seen after primary radical surgery. They occur more often when patients are treated with primary radiation therapy. Because of the gradual and progressive obliterative endarteritis produced by irradiating tissue, complications resulting from ischemic changes (e.g., cystitis, proctitis, enteritis, colpocleisis, pyelonephritis) can be seen many years after radiation treatment. Late onset of complications after primary radical hysterectomy and pelvic lymphadenectomy are unusual. These points are especially important when selecting a method of primary treatment for young women.
Psychological Benefits
There are probably important psychological benefits of primary treatment with radical surgery compared with radiation therapy. Most patients prefer to have the tumor removed and are especially encouraged when the surgeon can report that “the cancer is out” and that no evidence of metastatic disease was found at operation. Radiation therapy carries an unfortunate connotation in some patients who feel that it is the treatment of last resort, that the tumor is still there (albeit treated), or that irradiation will cause other cancers. All gynecologic surgeons have heard the disappointment patients express when they are told that they cannot be treated with an operation. Some patients continue to request an operation even after they have completed radiation therapy. It is paramount to council patients thoroughly and outline the many advantages modern radiation therapy may provide in select cases.
Justification for Pelvic Lymphadenectomy
Historically, there was competition between gynecologic surgeons who advocated radical vaginal hysterectomy without lymphadenectomy and those who advocated radical abdominal hysterectomy with lymphadenectomy. The advocates of extensive vaginal hysterectomy without lymphadenectomy (the Schauta-Amreich-Navratil operation) argued that their patients had fewer postoperative complications (especially urinary fistulae), a lower operative mortality rate, and a cure rate that was almost equal to that achieved by an abdominal operation that included lymphadenectomy. Furthermore, they pointed out that pelvic lymphadenectomy is an incomplete operation, at best, in that the removal of all pelvic lymph nodes that can possibly be involved with metastatic tumor is technically not feasible in many cases. This is especially important for those inferior gluteal nodes that are located in the region of the ischial spine, as pointed out by Reiffenstuhl. However, even Navratil stated in 1965 that “indications for the Schauta operation must take the lymph node problem into account.” Later, he performed extraperitoneal pelvic lymphadenectomy with the Schauta operation in all stage I and II cases that were locally advanced, as did Mitra, the Indian gynecologist who performed the pelvic node dissection through a bilateral extraperitoneal abdominal incision after radical vaginal hysterectomy.
In recent years, the operative mortality and complication rates in patients who have undergone radical abdominal hysterectomy and bilateral pelvic lymphadenectomy have significantly decreased. Operative mortality and fistulae occur in less than 1% and approximately 2% of patients, respectively. Therefore, one purported disadvantage of radical hysterectomy (high morbidity) has essentially been removed, and the surgeon can concentrate on the question of whether lymphadenectomy adds anything to the possibility of cure. If a pelvic lymphadenectomy is not done in patients who have a radical hysterectomy for cervical cancer, at least 15% to 20% of patients (those with positive nodes) will be inadequately treated for their disease (unless perhaps all patients receive postoperative pelvic irradiation). In our judgment, it is better to do a pelvic lymphadenectomy in all eligible patients and then give postoperative radiation therapy selectively than to avoid a lymphadenectomy and give postoperative radiation therapy to all patients.
It is the opinion of some that pelvic lymphadenectomy is of no value in those 80% to 90% of patients who have negative lymph nodes. We believe that lymphadenectomy is helpful in achieving an adequate central dissection around the cervical tumor, the most important part of the operation. This is especially true of that part of the lymphadenectomy that involves removal of tissue from around the hypogastric vessels, from the obturator fossa, and from the lower presacral region. Admittedly, dissection of lymph nodes from the common iliac vessels and from the paraaortic region does not add to the completeness of the central dissection. Removal of these and other nodes, however, is helpful in prognosis and in identifying patients at greater risk for persistent disease who might receive adjuvant postoperative radiation therapy to the pelvis and perhaps to extended fields along the aorta. Although we seldom dissect and remove the highest paraaortic lymph nodes, we do remove the lower paraaortic nodes around and just above the aortic bifurcation. If pelvic lymph nodes involved with tumor are found during the operation, a concerted effort is made to do a more complete paraaortic dissection. Although it is possible for paraaortic nodes to be directly involved without involvement of pelvic nodes, this is rare. For the group of patients who usually would be chosen for treatment with primary radical surgery,
routine extensive paraaortic lymph node dissection would not result in a therapeutic benefit very often. Podczaski and coworkers found positive paraaortic lymph nodes in 7 of 52 patients (13.4%) with stage IB and IIA disease. Twenty-eight of the 52 patients, however, had bulky tumors greater than 5 cm in greatest diameter. Currently, such patients are considered by many gynecologic oncologists not to be appropriate candidates for treatment with primary radical surgery. Patsner and coworkers performed paraaortic lymph node sampling in patients with small (tumor ≤3 cm) stage IB cervical cancer. Only 2 of the 125 patients who underwent radical hysterectomy, bilateral pelvic lymphadenectomy, and paraaortic node sampling had metastases to the paraaortic nodes. No patient had gross paraaortic nodal involvement, and both patients with microscopic paraaortic nodal metastases had grossly positive pelvic nodal involvement. These investigators recommended that paraaortic sampling in patients with small stage IB cervical tumors be restricted to patients with suspicious or positive pelvic or paraaortic nodes. The paraaortic region should be carefully palpated and any enlarged or firm nodes removed, but the gynecologic surgeon should be aware of the added morbidity associated with comprehensive paraaortic lymphadenectomy.
A study reported by Downey and colleagues provides indirect evidence that postoperative pelvic irradiation is more effective in controlling disease after pelvic lymphadenectomy has removed clinically positive lymph nodes that contain metastatic tumor. The amount of irradiation required to eliminate tumor in lymph nodes is directly related to the volume of tumor present (
Table 51.6). Thus, removing the larger nodes involved with tumor increases the probability of control of tumor with irradiation. Patients in this study who underwent resection of large positive pelvic nodes followed by postoperative extended-field irradiation had a surprisingly high 5-year recurrence-free survival rate of 51%. The advantages of surgical debulking of positive lymph nodes also were discussed by Potish and coworkers in a later study from the same center. No patient with unresectable pelvic nodes survived 5 years. In 84% (49/58) of cases with grossly positive pelvic nodes, the nodes were able to be debulked. The 5-year actuarial relapse-free survival rates were the same for patients with only microscopically involved pelvic node metastases (56%) and for patients with grossly involved but surgically resected pelvic node metastases (57%). All patients with positive pelvic nodes received postoperative irradiation to the pelvis and paraaortic nodes. These authors believe that surgical debulking of grossly involved pelvic lymph nodes to microscopic residual disease may improve the chance of control with postoperative irradiation.
PERTINENT PELVIC ANATOMY ARTERIAL AND VENOUS ANATOMY
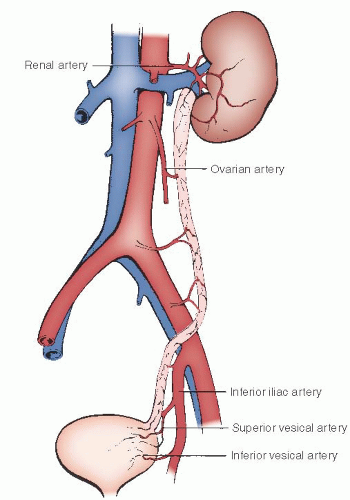
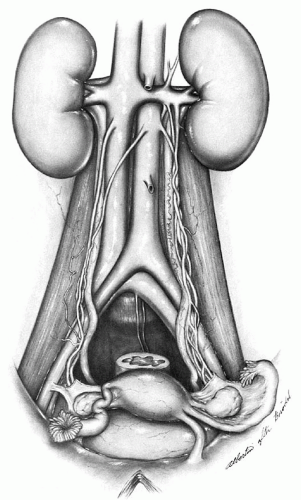
Although the lower portions of the aorta and vena cava are frequently incorporated into the operative field of the pelvic lymphadenectomy, the major operative dissection includes the common iliac, external iliac, and hypogastric (also known as the internal iliac) arteries and veins and their various branches and tributaries. The abdominal aorta emerges through the aortic hiatus of the diaphragm at the lower border of the last thoracic vertebra and descends along the ventral surface of the vertebral column, where it bifurcates into the left and right common iliac arteries at the fourth lumbar vertebra (
Fig. 51.10). This is an important anatomic landmark because the bifurcation at L4 lies directly beneath the umbilicus in most cases. Therefore, an abdominal midline incision that provides surgical exposure to the lower aorta needs to be extended somewhat above the umbilicus. The right common iliac artery crosses the upper portion of the left common iliac vein at the aortic bifurcation. This segment of the venous drainage of the left side of the pelvis
joins with the right common iliac vein to form the vena cava, which lies directly along the right side of the aorta and on the right lateral side of the bodies of the lumbar vertebrae in its retroperitoneal course through the abdomen.
Both common iliac arteries continue along the medial border of the psoas muscle to the pelvic brim, where they divide into external iliac and hypogastric vessels. As shown in
Figure 51.10, this important vascular division marks the site where the ureters enter the pelvis from the abdomen, usually overlying the terminal end of the common iliac artery on the left and commonly crossing the actual bifurcation of the artery on the right. Both external iliac arteries pass beneath the inguinal ligament to proceed into the leg as the femoral artery. The external iliac artery makes no direct vascular contribution to the pelvis, although there is a fairly consistent arterial branch to the ureter from the midportion of the common iliac artery.
The external iliac vein emerges from beneath the inguinal ligament, where it courses along the lateral pelvic brim on the medial side of the artery until it reaches the proximal segment. Here, the vein passes directly beneath the artery at the bifurcation of the common iliac artery and then passes along the lateral side of the upper half of the artery. It then joins the left common iliac vein to become the inferior vena cava at the fifth lumbar vertebra. In dissecting the lymph nodes along the external iliac vessels, these anatomic landmarks are important to avoid trauma to the wall of the vein as it deviates from the medial to the lateral side of the arterial tree.
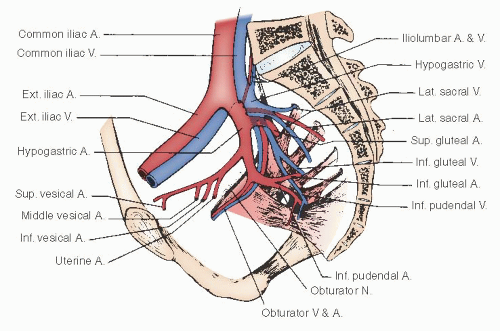
The hypogastric artery provides the major blood supply to the pelvic viscera. For descriptive purposes, it is conveniently divided into an anterior and a posterior division. The important branches of the hypogastric artery are shown in
Figures 51.11 and
51.12.A fairly consistent arterial branch to the ureter arises from the hypogastric artery near the common iliac bifurcation. This vessel passes medially to the ureter and should be preserved, if possible, during the dissection of the hypogastric vessels. The posterior division of the hypogastric artery continues beneath the coccygeus muscle through the ischiorectal fossa, where it becomes the internal pudendal artery to supply the perineum and vulva.
The major blood supply to the pelvic viscera is derived from the anterior division of the hypogastric artery.
Figure 51.11 shows the anterior division, which gives off the uterine artery before continuing along the posterolateral pelvic wall to supply the superior and inferior vesical branches to the bladder. The anterior division then continues as the obliterated umbilical artery as it passes cephalad along the inferior surface of the rectus muscle to the umbilicus. In dissecting along the hypogastric artery in a caudad direction, the uterine artery is the first vessel encountered; it emanates from the medial side of the vessel. Passing more inferiorly and medially is the middle hemorrhoidal artery, which supplies a major segment of the rectum and communicates with the superior hemorrhoidal (from the inferior mesenteric) and the inferior hemorrhoidal (from the internal pudendal) arteries.
The hypogastric vein and its tributaries course along the pelvic floor and medial side of the artery to drain the pelvis in close relation to the arterial blood supply. Its extensive anatomic variations and its location along the pelvic sidewall and floor place these tortuous, thin-walled veins in a precarious and vulnerable position for trauma during deep dissection of the pelvis. As shown in
Figure 51.12, the delicate tributaries of the trunk of the hypogastric vein extend into sacral foramina and pass beneath nerve fibers and muscles within the pelvis, so that their visualization during the dissection of the pelvis frequently is obscured. The continuation of the hypogastric vein, in association with the artery, beneath the coccygeus muscle is a frequent site of bleeding when dissection is undertaken along the pelvic floor. When this occurs, it is difficult to identify the vessel because it retracts beneath the margins of the muscle.
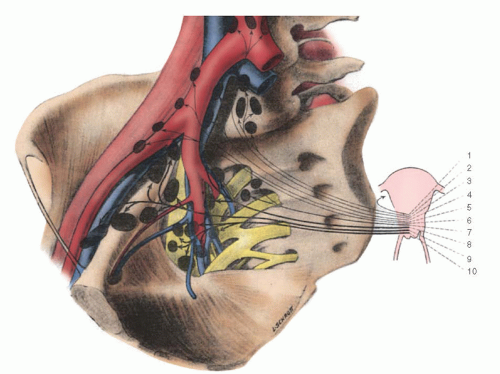
The profuse collateral blood supply to the ureter is an important anatomic safeguard that protects its pelvic segment from ischemic necrosis as a result of radical hysterectomy (
Fig. 51.13). The ureter has the advantage of a multiple-source blood supply. This favorable collateral circulation permits interruption of small arteries and veins deep in the pelvis during extensive dissection of the base of the broad ligament without producing a significant incidence of ischemic necrosis and fistula formation. The freely anastomosing arterial and venous network that courses along the longitudinal surface of the ureter in its adventitial layer is supplied in its superior segment by branches from the renal and ovarian arteries. The middle segment of the ureter derives its blood supply directly from aortic branches and from a vessel from the common iliac artery. As the ureter enters the pelvis and courses along the lateral pelvic wall, it receives arterial branches from the uterine, vaginal, middle hemorrhoidal, and vesical arteries. As it approaches the trigone of the bladder, it has a rich arteriovenous collateral circulation from the arterial branches to the vagina and base of the bladder. Protection of this important vascular network is important for the integrity of the terminal ureter during extensive dissection of the cardinal ligament. Preservation of the lateral aspect of the posterior segment of the vesicouterine ligament has been recommended to ensure adequate vascularity to the terminal segment of the ureter, but we have encountered no difficulty in removing this tissue and have no hesitation in doing so to enhance the adequacy of the central dissection.
Lymphatic Anatomy
The lymphatic drainage of the pelvis follows the course of the arterial and venous blood supply. Although there are multiple variations in the lymphatic anatomy of the pelvis, in general, lateral, superior, medial, and inferior lymph nodes and communicating lymphatic channels surround the common iliac, external iliac, and hypogastric vessels. One of the important pathways of the pelvic nodes and thin-walled lymphatics that drain the upper vagina, cervix, and uterus courses along the posterior aspect of the endopelvic fascia. Here, the pelvic nodes pass through the uterosacral ligament area and terminate in lymph nodes along the lateral aspect of the sacrum. These nodes communicate freely with lymphatic channels from the bifurcation of the common iliac artery near the lateral sacral and ischiosacral fossae. These can be difficult nodes to resect because they are closely attached to the thin-walled tributaries of the hypogastric vein. In dissecting the nodes from the
bifurcation of the common iliac vessels, care must be taken to avoid injury to the hypogastric vein, which extends from beneath the artery on the medial side in this area.
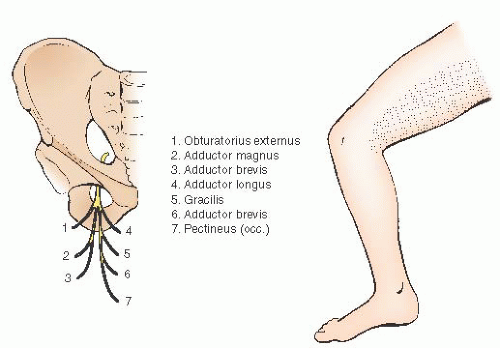
The most direct lymphatic drainage of the cervix and upper vagina is through the lateral parametrium (cardinal ligament) to the hypogastric and obturator lymphatics. Because of the presence of obscure obturator veins and multiple venous tributaries from the hypogastric vein along the pelvic floor, the obturator dissection can be associated with troublesome venous bleeding. Injury also can occur to the obturator nerve, which arises from the anterior division of the second, third, and fourth lumbar nerves; enters the pelvis through the psoas muscle; and runs along the lateral pelvic wall in the obturator fossa to exit the pelvis through the obturator foramen along with the obturator vessels. It is a motor nerve to the adductor muscles of the thigh and is the only motor nerve that arises from the lumbar plexus without innervating any of the pelvic structures. Damage to the obturator nerve produces not only motor impairment to the adductor muscles but also sensory loss along the medial aspect of the thigh (
Fig. 51.14). Deep dissection posterior to the obturator nerve can be complicated by bleeding from the tributaries of the hypogastric and obturator veins, so dissection in this area must be done with great care, using clips or vessel sealers on the small vessels and compression for troublesome venous bleeding.
Reiffenstuhl, in his classic study of the lymphatics of the female genital organs, described efferent lymph channels from the cervix to the interiliac lymph nodes, the lateral and medial external iliac lymph nodes, the lateral and medial common iliac lymph nodes, the sacral lymph nodes, the subaortic lymph nodes, the aortic lymph nodes, the superior gluteal lymph nodes, the inferior gluteal lymph nodes, and the rectal lymph nodes. Of these, the inferior gluteal nodes are not technically possible to remove with the standard approach. This is because the nodes lie around the ischial spine in proximity to the inferior gluteal artery and pudendal artery and nerve. An imposing network of veins also surrounds the inferior gluteal nodes. They are thin walled, easy to damage, difficult to expose, and difficult to control when damaged. Reiffenstuhl’s concepts of the lymphatic drainage of the cervix are partially shown in
Figure 51.15.Sentinel lymph node studies in women with cervical cancer are also adding greatly to our knowledge of the lymphatic drainage in vivo (
Fig. 51.16A), and identification of SLNs with blue dye (
Fig. 51.16B), fluorescent indocyanine green (ICG) (
Fig. 51.16C), and technetium injections is feasible, with very low morbidity. These SLNs can be submitted for ultrastaging with immunohistochemistry staining and may help in the postoperative management of cases. Further evaluation and validation of SLN techniques is leading many investigators to recommend the routine use of SLN with pelvic nodal dissection and even consider an SLN algorithm as a comprehensive strategy to evaluate nodal metastasis. Recent studies have strongly suggested that SLN identification can potentially replace complete pelvic lymphadenectomy in select early-stage (I) cervical cancer. The SLN algorithm may help reduce the total operative time as well as some perioperative complications and long-term complications, such as postoperative leg lymphedema, which is occasionally seen in patients undergoing pelvic lymphadenectomy. The most common injection sites for SLN mapping are shown in
Figure 51.16D.
EVOLUTION OF THE CLASSIFICATION SYSTEMS OF RADICAL HYSTERECTOMY AND PELVIC LYMPHADENECTOMY
There are several variations of hysterectomy used in the management of cervical carcinoma. The description of the five classes of hysterectomy by Piver and colleagues has found general acceptance and was used for many years. The first three classes are used in the primary treatment of cervical carcinoma, whereas the last two classes are generally reserved for patients
with recurrent disease. The class I hysterectomy is a simple extrafascial total hysterectomy. It is used as primary treatment for stage IA1 disease and after concurrent chemotherapy and radiation therapy for stage IB2 and bulky stage
IIA cervical carcinoma. The class II hysterectomy (
Fig. 51.8), also known as a modified radical hysterectomy, removes a more generous vaginal cuff, ligates the uterine artery on the medial side of the ureter (but does not dissect the ureter from the vesicouterine ligament), and removes the inner one third to one half of the cardinal ligament. As previously stated, some authors recommend the performance of the class II hysterectomy for stage IA2 cervical carcinoma. The class
III operation is the classic Meigs procedure, with removal of all of the parametrium and paravaginal tissue in addition to the pelvic lymph nodes (
Fig. 51.8). A more extensive procedure is performed in the class IV radical hysterectomy, in which the ureter is completely dissected from the cardinal and vesicouterine ligaments, the superior vesical artery is sacrificed, and three fourths of the vagina is removed, as well as the uterus and parametria, along with a complete lymphadenectomy. A far more extensive procedure is done with the class V radical hysterectomy, in which the terminal ureter or a segment of the bladder or rectum is removed along with the uterus, parametria, adnexa, and pelvic lymph nodes.
Although many techniques emphasize a more or less extensive dissection in one phase of the operation or another, the management of the parametria and the dissection of the pelvic lymph nodes appear relatively uniform. Because the most serious complication of this procedure is related to ureteral fistulae and stenosis, many modifications have been undertaken to ensure an adequate blood supply to the terminal ureter. We agree that the terminal ureter must have a good blood supply and believe that this can be accomplished without jeopardizing the adequacy of the central dissection. The classic radical hysterectomy with wide resection of the parametrium, dissection of the terminal ureter from the vesicouterine ligament, and wide resection of the uterosacral ligaments, upper 2 to 3 cm of vagina, and paravaginal tissues, along with a thorough pelvic lymphadenectomy, is the traditional procedure that is used in our institution.
The major focus of the operation is the adequacy of the central dissection. The central cervical tumor must be removed with an adequate margin of uninvolved normal tissue around it. This is the most crucial point in the success of the operation and has been emphasized by many of the famous pelvic surgeons of former years, especially Parsons and Navratil. The central dissection can be facilitated by developing the pelvic spaces and using proper planes for dissection. Correct
dissection along natural rather than artificial connective tissue planes and correct development of the pelvic spaces (paravesical, pararectal, vesicocervical, and rectovaginal) avoid unnecessary injury to pelvic vessels, keep blood loss to a minimum, and facilitate an adequate central dissection (
Fig. 51.17). The central dissection also is facilitated by a complete removal of the contents of the obturator fossa (except the obturator nerve) so that branches of the hypogastric artery and vein in the cardinal ligament are clearly visible and can be dissected away from their attachments to the lateral pelvic sidewall.
The importance of an adequate central dissection also was emphasized by Girardi and coworkers. By studying surgical specimens processed according to the giant-section technique of Burghardt and Pickel, parametrial lymph nodes were found in 280 (78%) of the 359 surgical specimens from radical hysterectomies. Metastases to parametrial nodes were found in 63 (22.5%) of these 280 specimens. The lymphatic drainage from the cervix to the pelvic lymph nodes runs through the parametrium, and deposits of tumor often are found there. An adequate central dissection must include removal of a wide margin of parametrial tissue around the central tumor and total removal of the parametria from the bladder, the rectum, and the lateral pelvic wall because positive lymph nodes can be found in the lateral as well as medial parametrium.
When a large vaginal cuff must be removed because of a bulky cervical tumor or involvement of adjacent vaginal mucosa, starting the operation from below may facilitate the central dissection. Sometimes, a bulky lesion is excised and fulgurated transvaginally. The formation of the vaginal cuff is done in a manner similar to that in the Schauta-Amreich procedure. The vaginal incision is made around the entire circumference of the vagina, mobilizing the vaginal cuff from paravaginal tissue. Further dissection into the paravesical space, vesicocervical space, and rectovaginal space from below may be easier than from above.
Superior to the midcommon iliac arteries and in the paraaortic region, lymph nodes are sampled in selected patients (usually stage IB1 and IIA). A special effort is made to remove any nodes that look or feel suspicious. The paraaortic lymph nodes then are sent for frozen section analysis. Approximately 5% to 10% of stage IB patients have paraaortic lymph node metastasis. Metastasis to the paraaortic lymph nodes is considered by many gynecologic oncologists as a contraindication to radical hysterectomy. These patients are currently being treated with extended-field radiation therapy with concurrent chemotherapy.
In recent years, Querlu and Morrow suggested a modernization of the classic classification system, with a main emphasis on the role of a nerve-sparing radical hysterectomy (type C1) and on three-dimensional topography of radical pelvic surgery. Simply put, a type B resection is equivalent to a class II hysterectomy, a type C1 resection is equivalent to a class III hysterectomy with nerve sparing, and a class C2 hysterectomy is equivalent to a more radical class III operation with disregard to hypogastric and pelvic splanchnic nerves.
RADICAL ABDOMINAL HYSTERECTOMY SURGICAL TECHNIQUE
Preoperative Evaluation and Preparation
After the initial history and physical examination have indicated the possibility of primary treatment with radical hysterectomy and pelvic lymphadenectomy, the usual preoperative evaluation common before any extensive operation is indicated. We also recommend a chest x-ray to screen for cardiac or pulmonary disease as well as the very low risk of pulmonary metastases.
Contrary to the frequent practice of doing all possible tests on every patient, it is our practice to be selective and to do only those tests and procedures that are expected to yield useful information. It is not necessary (and indeed may be inappropriate) to subject every patient to a long list of preoperative procedures that are expensive and exhausting and have little, if any, expectation of providing useful information. Indeed, it is most unfortunate when a test or procedure yields questionable or suspicious findings that require one or more additional studies that, after delay, discomfort, and expense, turn out to be negative or even perhaps nondiagnostic. Good judgment from an experienced clinician is usually the most helpful.
A young, healthy patient with a small cervical lesion requires an admission history and physical examination, chest radiograph and routine laboratory studies, and anesthesia consultation. If the patient is older, has medical complications, or has a larger or undifferentiated cervical lesion, the preoperative workup and preparation may be more involved and thorough. Although an intravenous pyelogram provides good visualization of the course and number of ureters, if radiologic evaluation of the abdomen and pelvis is being considered, most surgeons obtain a CT scan of the abdomen and pelvis with contrast, which also shows the course and number of ureters and also may provide additional information about nodal or other metastases. However, because of the uncertainty of enlarged nodes on CT scan, patients with clinical stage IB1 disease and enlarged pelvic nodes on CT scan would not make us cancel the surgical approach in favor of radiation therapy. These nodes need to be evaluated further, possibly with a surgical resection, biopsy, or PET scan.
Larger lesions or those more likely to have metastases may be investigated by pelvic and abdominal CT scan or magnetic resonance scans, but we have rarely found those to be helpful in women with small cervical cancers. They may be most helpful when the clinician is undecided regarding whether primary surgery or radiation is the best management option—a clean, normal study may be reassuring that primary surgery is the way to go, whereas suspicious, enlarged nodes in a woman with a 4-cm cervical lesion help make the decision to recommend radiation. Likewise, cystoscopy or proctoscopy is rarely indicated or helpful in the preoperative evaluation of early-stage cervical cancer. As much as possible
should be done on an outpatient basis before admission to the hospital.
Bowel Preparation
In our institution, patients are asked to start a liquid diet 24 hours before surgery. They also are given a mechanical bowel preparation if significant peritoneal adhesions are anticipated or there is a history of previous major pelvic surgery or radiation. An intestinal tube for suction is not necessary.
Positioning and Incision
Multiple approaches to performing a radical abdominal hysterectomy and bilateral pelvic lymphadenectomy have been described. The traditional transperitoneal approach has been used in our institution for many years, with satisfactory results. The transverse Maylard or Cherney incision is used by some, whereas others prefer midline incisions. Orr and Scribner have reported shorter hospital stays when a Pfannenstiel incision is used. Currie has presented and published a technique using a transverse cosmetic incision with vertical fascial entry for selected patients.
After anesthesia is induced, some surgeons prefer that the patient be placed comfortably in stirrups, with the buttocks brought to the edge of the “broken” table. Pneumatic compression devices are placed on both lower extremities, and the knees are separated approximately 90 degrees. The thighs are elevated only 15 to 20 degrees relative to the abdomen. Care is taken to avoid pressure on the peroneal nerves in the legs. Proponents of this position claim several advantages. There is less strain on the patient’s lumbosacral spine when the thighs are slightly flexed. This is especially important for patients with lumbosacral back problems. It is possible to have a second assistant stand at the foot of the table between the patient’s legs. His or her participation in the operation is greatly facilitated by being closer to the operative field. Finally, in this position, the urethral orifice, vaginal introitus, and anal orifice are all available for instrumentation in case this is necessary to clarify anatomy.
After the patient is positioned on the operating table, a careful rectovaginal-abdominal pelvic examination is done. This can be followed by cystoscopy or proctosigmoidoscopy if desired. If hair needs to be removed, it is preferable to use an electric hair trimmer rather than a razor. The skin is prepared from the rib margin to the midthigh, with special attention given to the umbilicus, perineum, and vagina. The patient is draped, a transurethral Foley catheter is inserted into the bladder, and the operation is begun. If an SLN injection is planned, the injection is done at the time of exam under anesthesia just before the patient is prepped and draped.
When operating abdominally, the exposure achieved depends on the choice of incision, the method of retracting, the placement and intensity of overhead lights, and the participation of willing and skillful assistants. Suction should be available to keep the field dry. Dry sponges are likely more traumatic to delicate serosal surfaces and other tissues. It usually is possible (and always desirable) to keep the number of clamps in the operative field to an absolute minimum. If the field is cluttered with clamps, the operator cannot see well to operate. There is sometimes a tendency for gynecologic surgeons to use instruments that are too short. Pedicle clamps, tissue forceps, dissecting scissors, needle holders, and all other instruments must be longer when operating deep in the pelvis and when operating on obese patients. The handles of the instruments must come all the way out and above the level of the incision so that they do not interfere with the operator’s vision.
Evaluation at Laparotomy
As stated, the operation is initiated through a low-transverse (Maylard, Cherney, Pfannenstiel) or low midline incision; the same exploratory strategy should apply if a minimally invasive approach is being utilized. In most patients, the umbilicus identifies the location of the bifurcation of the aorta; therefore, extension of the incision approximately 2 to 3 cm above the umbilicus is recommended for adequate exposure of this area if a lower midline incision is used. The midline incision is protected by a moist pack beneath each arm of the self-retaining retractor to avoid excessive compression of the epigastric vessels that course beneath the rectus muscles. In case of a lengthy operative procedure, the mechanical retractors are released at periodic intervals to improve circulation through the abdominal musculature. The bladder is decompressed by an indwelling catheter throughout the procedure to facilitate exposure and maintain an accurate record of urine output.
Before initiating the pelvic procedure, the abdominal viscera and parietal peritoneum of the abdominal cavity are evaluated meticulously for possible evidence of metastatic tumor. The superior and inferior surfaces of the liver are carefully palpated, as is the region of the celiac plexus. The undersurface of the diaphragm is particularly vulnerable to metastases, especially the right hemidiaphragm, where the paraaortic lymphatics pass from the abdominal cavity into the mediastinum. The mesentery of the large and small bowel and the serosal surface of the bowel along with the omentum should be examined carefully for evidence of metastatic tumor. The kidneys are examined, and the retroperitoneal space along the aorta and vena cava is palpated assiduously because these are the major sites of extrapelvic spread of cervical cancer. It is well known that 15% or more of paraaortic node metastases are occult; therefore, even the most unsuspecting node should be removed and evaluated histologically by frozen section study for possible metastatic tumor. If there is histopathologic evidence of unsuspected, metastatic tumor in a paraaortic lymph node, we generally do not proceed with a radical hysterectomy, and the operation is abandoned. These patients are currently being treated with concurrent chemotherapy and extendedfield radiation therapy.
Peritoneal washings for cytologic examination usually are not obtained because the yield is low and the prognostic significance is undetermined.
At this point in the procedure, any adhesions in the pelvis are lysed, and the intestines are placed in the upper abdomen and held there with moist packs. A suitable self-retaining retractor can be used. If Bookwalter, Turner-Warwick, or Balfour retractor is used in a lower midline incision, care must be taken to avoid compression of the femoral nerves by the lateral blades.
An evaluation of the extent of the pelvic tumor is undertaken at this time by examining the course of the lymphatic drainage of the pelvis, which is carefully palpated along the pelvic vessels. When enlarged or clinically suspicious nodes are found, they are removed and immediately sent for frozen section study while further evaluation of the pelvis is undertaken. The paravesical and pararectal spaces are important anatomic landmarks. When developed, they provide an opportunity for thorough exploration of the intervening base of the broad ligament (
Fig. 51.17). Tumor can extend into the base of the broad ligament without detection of anatomic evidence of disease before operation. This step, therefore, is a safeguard in determining the possible extension of tumor beyond the cervix and into the immediate paracervical tissues. When there is evidence of extracervical disease, we may abandon the surgical
procedure unless there is clear evidence that the disease can be removed cleanly. In either case, full pelvic irradiation is indicated. Certainly, the lateral pelvic wall must be free of tumor. When the central tumor is clearly resectable, we do not hesitate to complete the operation, even if there is evidence of metastatic disease in the pelvic lymph nodes.
A decision must be made about conservation or removal of the tubes and ovaries before the pelvic planes and spaces are developed. If normal ovaries are conserved in premenopausal patients, the tubes usually may be removed. After the round ligaments are clamped, cut, and ligated, the utero-ovarian ligaments and medial fallopian tubes are clamped and doubly ligated. The infundibulopelvic ligaments are carefully mobilized, and the adnexal organs are packed out of the operative field with the intestines.