Bronchopulmonary Dysplasia
Timothy D. Murphy
Bronchopulmonary dysplasia (BPD)—sometimes called chronic lung disease of infancy— is a disorder of lung injury and repair in infants who developed respiratory failure after birth and is characterized by persistent respiratory signs and symptoms. The pathogenesis of BPD is related to prematurity and the treatment of the accompanying early respiratory failure; the treatment interacts with the immature developing lung, causing further injury and delayed repair. The injurious effects of treatment include barotrauma from positive-pressure ventilation and oxygen toxicity. However, other therapies developed in the past 40 years have led to a different clinical, histological, and radiographic presentation and have redefined the condition. For a more detailed discussion of the pathogenesis, risk factors and prevention of BPD see Chapter 59. 
New histological findings reflect an evolution in treatment and hence a change in the patient population that develops the disease; now BPD is found in very premature infants of a younger postconceptual age who are treated with surfactant. The differences between the original definitions of BPD and what has been termed the “new BPD” will be discussed in the following sections.
 EPIDEMIOLOGY, SPECIFIC POPULATIONS AT RISK, AND ASSOCIATED DISORDERS
EPIDEMIOLOGY, SPECIFIC POPULATIONS AT RISK, AND ASSOCIATED DISORDERS
Although the use of surfactant replacement therapy led to a decrease in the incidence of BPD, the overall incidence of this disorder has not changed much over the past decade.4 Neonatal centers vary widely in reported incidence of BPD, which is about 20% of all ventilated premature newborns.5
The risk of BPD increases with lower birth weight, with an incidence of up to 85% in neonates weighing between 500 g and 699 g; most infants developing BPD are premature, and 75% of affected babies weigh less than 1000 g at birth.6
BPD remains primarily a disorder affecting premature infants, although various definitions have been developed to include infants with other early lung injuries such as from group B streptococcus pneumonia, meconium aspiration syndrome, or ventilator-induced injury in term infants with pulmonary hypertension. The term chronic lung disease of infancy has been coined to include infants with the shared characteristics of lung injury, barotrauma, and oxygen toxicity, regardless of gestational age. Thus, by most definitions, the smaller and younger the premature infant, the greater the risk of developing BPD. In 2000, an NIH-sponsored workshop on BPD reviewed the current definition of BPD in the hopes of better defining diagnostic criteria for this illness (Table 513-1).14 Factors associated with premature delivery, such as younger or older maternal age, lower socioeconomic status, African American descent, prenatal infection with Ureaplasma ureolyticum, and chorioamnionitis, lead to a higher risk of BPD.
 PATHOPHYSIOLOGY AND GENETICS
PATHOPHYSIOLOGY AND GENETICS
Due to new treatments for prematurity and surfactant deficiency, the mechanisms of injury and repair are evolving. In “classic” bronchopulmonary dysplasia (BPD), the primary processes are oxygen toxicity and barotrauma. In “new” BPD, although oxygen toxicity and barotrauma are still important, arrested development of the lung, with diminished number and complexity of airways and alveoli, is present. The mechanisms of injury particular to the old and the new BPD are not mutually exclusive; therefore, the mechanisms will be discussed in general terms, with a focus on current trends in care and outcomes.
Table 513-1. Definition of Bronchopulmonary Dysplasia: Diagnostic Criteria
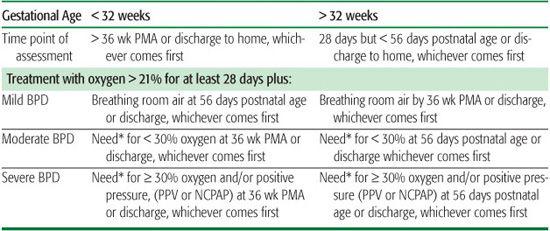
Infants treated with oxygen over 21% or with positive pressure for nonrespiratory disease (eg, central apnea or diaphragmatic paralysis) do not have BPD unless they also develop parenchymal lung disease and exhibit clinical features of respiratory distress. A day of treatment with oxygen greater than 21% means that the infant received oxygen over 21% for more than 12 hr on that day. Treatment with oxygen greater than 21% and/or positive pressure at 36 wk PMA, or at 56 d postnatal age or discharge, should not reflect an “acute” event, but rather the infant’s usual daily therapy for several days preceding and following 36 wk PMA, 56 days postnatal age, or discharge. BPD, bronchopulmonary dysplasia; NCPAP, nasal continuous positive airway pressure; PMA, postmenstrual age; PPV, positive-pressure ventilation.
*There is no defined or accepted approach to confirm the oxygen requirement at the time of assessment.
Adapted from Jobe AH, BancalariE. Bronchopulmonary dysplasia. Am J RespirCrit Care Med.2001; 163(7): 1723-1729.
Oxygen toxicity is caused when the fraction of inspired oxygen (FiO2) is greater than 21% for a sustained period; higher concentrations and time of exposure to oxygen are associated with worse injury.15,16 Oxygen toxicity is mediated by reactive oxygen and nitrogen species (ROS and RNS, respectively). Vulnerability to oxidant injury is increased in premature infants, as antioxidant enzyme systems are not yet matured and exogenous antioxidants such as vitamin C and E are wanting in the premature newborn. Hyperoxia induces movement of inflammatory cells into intravascular and interstitial spaces, damages vascular endothelium, and changes the makeup of alveolar pneumocytes.
Higher pressures or volumes used during mechanical ventilation damage the airway and parenchyma.21,22 Larger volumes increase the lung damage, and asynchrony of inspiratory and expiratory pressures in the mechanically ventilated newborn are associated with increased risks for intraventricular hemorrhage and airleak.23 Ventilatory strategies to avoid barotrauma have been developed and reduce the incidence of lung injury and BPD, although none prevent it entirely.24
Genetic differences may account for some of the variability in the presentation of BPD. Neonates with certain genetic defects are more likely to be born early and to therefore develop BPD. Sex and race affect the severity of the respiratory distress syndrome (RDS) in premature infants and thus affect the incidence of BPD. Genetic differences of surfactant protein B have been associated with BPD,25 and genes that might modify BPD have been identified.26
 CLINICAL FEATURES AND DIFFERENTIAL DIAGNOSIS
CLINICAL FEATURES AND DIFFERENTIAL DIAGNOSIS
The clinical features of BPD include respiratory distress characterized by tachypnea with small tidal volumes, retractions, and use of accessory muscles of respiration with a paradoxic breathing pattern; auscultation may reveal rales, rhonchi, or wheezes. Consistent with classic pathophysiology, the patient is hypoxic, which reflects the heterogeneous distribution of disease with regional differences in lung compliance and airway caliber, leading to ventilation-perfusion mismatching.  The evolution of the radiograph in classic BPD, originally described by Northway3 as demonstrating cysts alternating with strands of increased density, is less commonly seen now. The new BPD is described as evolving from nearly clear lung fields initially to a hazy and diffuse pattern of opacification and then into more evenly distributed coarse interstitial opacities without cystic lesions.28
The evolution of the radiograph in classic BPD, originally described by Northway3 as demonstrating cysts alternating with strands of increased density, is less commonly seen now. The new BPD is described as evolving from nearly clear lung fields initially to a hazy and diffuse pattern of opacification and then into more evenly distributed coarse interstitial opacities without cystic lesions.28
 DIAGNOSTIC EVALUATION
DIAGNOSTIC EVALUATION
The diagnosis of bronchopulmonary dysplasia (BPD) is uncomplicated and is based on historical facts; the primary goal of diagnostic evaluation is to determine if any other processes are present and complicating recovery, as recovery is eventually the more common course of events with BPD. Evaluation of an infant with BPD focuses on identifying alternative causes of disease or worsening symptoms that might change therapy. If the patient is in respiratory failure, direct measurement of the partial pressure of carbon dioxide in blood, or indirect measurement using transcutaneous or exhaled levels of carbon dioxide, is important; relying on oxyhemoglobin saturation to measure the adequacy of ventilatory management poses a risk of missing hyper- or hypoventilation. Following extubation, arterial blood gases are rarely used; capillary or venous blood gases, or serum bicarbonate levels, used as an indirect measure of ventilation are used more frequently. Chest radiographs are monitored frequently during acute, early respiratory failure in a premature infant but are less useful in later management. Infants requiring prolonged mechanical ventilation of more than 100 days should have a formal evaluation for tracheotomy or for chronic ventilatory support.
 TREATMENT
TREATMENT
As chronic lung disease develops, strategies used for prevention are often the same as those used for treatment. The treatment of chronic lung disease often reflects a continuation of care initiated to prevent it. Treatment of BPD relies on promoting healing from lung injury; the difference between prevention and treatment strategies may blur in the care of a child with BPD. Hence, treatment of this disorder includes continued avoidance of oxygen toxicity, barotrauma, and lung inflammation. Additionally, therapy not proven to prevent BPD may be used more commonly later in treatment.
Oxygen Therapy
Chronic hypoxemia is a consequence of the ventilation-perfusion (V/Q) mismatching of BPD. It slows growth and increases the risk of developing pulmonary hypertension and dying. Oxygen therapy sufficient to prevent such problems is the treatment’s goal. Delivered by nasal cannula, flow is titrated to keep the SaO2 levels between 91% and 96%. Length of therapy is related to severity of lung disease—patients are commonly off oxygen by 6 to 10 months of age. Temporary setbacks due to intercurrent viral illnesses are common, but a persistent increased oxygen requirement suggests the presence of an ongoing insult to lung health.
Diuretics
Use of furosemide has been associated with improved lung mechanics in newborns, and radiographs have shown that diuretics can lead to improved gas exchange and resolution of rales and infiltrates. A relative fluid restriction in the first 4 days of life is associated with less severity of illness and development of BPD. Supplementation with potassium chloride (KCl) may be required to prevent development of a metabolic alkalosis from furosemide therapy.
Spironolactone and hydrochlorothiazide are commonly used (often in combination) because of improved safety and reduced potassium loss, but these may have less effect on lung mechanics and are less well studied. Mechanical ventilation and diuretic therapy may lead to hypochloremia and a contraction alkalosis. This in turn may be treated with NaCl supplements, triggering fluid retention and a further need for diuretics and making the discontinuation of diuretics more difficult. Sodium chloride, potassium chloride, and diuretics are not usually needed outside of the first few months of life and are usually discontinued before oxygen therapy. Diuretics may be required longer in infants who have associated cardiac failure or during episodes of worsening symptoms associated with acute infections.
Bronchodilators
Inhaled beta-agonists and anticholinergic agents can improve lung function in BPD, but it is not clear that such therapy leads to a more rapid improvement, a different ultimate outcome, or improvement in quality of life. Small studies have failed to demonstrate earlier weaning off of respiratory support or reduction in the incidence of BPD. Lacking data that bronchodilators are effective for all patients with BPD, their use is reserved for those who demonstrate evidence of reversible airway obstruction. For patients with clinical evidence of tracheobronchomalacia, the use of anticholinergic agents like ipratropium bromide may be indicated. Atropine sulfate is rarely used due to its excessive drying effects and the risk of plugging with inspissated mucus. Ipratropium may pose the same risk in a child with a tracheotomy or endotracheal tube—drug dosage delivered via endotracheal or tracheal tube may be much higher than that via mouthpiece or face mask. Excessive drying after antimuscarinic therapy may require the adjustment of dosage. 
Corticosteroids
The inflammatory nature of BPD suggests that anti-inflammatory therapy would prevent or treat the illness. Corticosteroids given early in the course of hyaline membrane disease demonstrated a reduction in BPD and earlier extubation, but larger multicenter studies did not reproduce such findings. Moreover, such studies demonstrated an increase in adverse neurodevelopmental outcomes. Consequently, steroids are no longer routinely used in the treatment of premature newborns.
Currently, in older infants with BPD, steroids sometimes are used to treat airway obstruction. The use of steroids in this older group of patients follows more the patterns of use for asthma exacerbations. The relative risk of steroids to neurodevelopment in older babies is unknown but presumedly lower than the risk in the newborn period.  In older infants who demonstrate persistent respiratory failure, steroids may be tried in an attempt to improve the long-term outcome or to achieve extubation. Chronic use of steroids is avoided; consideration of alternative therapies, even resuming mechanical ventilation, must be considered in infants unable to come off steroids.
In older infants who demonstrate persistent respiratory failure, steroids may be tried in an attempt to improve the long-term outcome or to achieve extubation. Chronic use of steroids is avoided; consideration of alternative therapies, even resuming mechanical ventilation, must be considered in infants unable to come off steroids.
Inhaled Corticosteroids
Given the generalized inflammatory nature of BPD, inhaled corticosteroids (ICS) have seemed a reasonable treatment option physiologically. In general, studies of ICS have shown improvements in specific pulmonary measures and less need for systemic steroids, long-term mechanical ventilation, or bronchodilator use. Clinically, ICS are used when a patient shows a pattern of reliance on systemic steroids or evidence of inflammatory airways disease that improves after initiating therapy. However, the development of BPD may not be a sensitive enough measure of a drug’s utility; multiple studies of ICS have shown little change in the incidence of BPD or oxygen requirements. 
Antiviral Therapy
Palivizumab (a humanized monoclonal antibody) therapy can reduce the incidence and severity of respiratory syncytial virus (RSV) infection in vulnerable children with BPD. Prophylactic use of palivizumab is routinely given to children under 2 years of age who have BPD and who require medical therapy in the 6 months prior to the onset of the RSV season. 
Palivizumab is sometimes given to older children up to age 4 who are still ventilator dependent or who have significant comorbidi-ties. Though studies demonstrating the value of this are lacking, deaths due to RSV in medically fragile children continue to prompt its use.
Other Pharmacological Therapies
Other medications commonly used in older children with BPD include long-acting beta-agonists, leukotriene inhibitors and methylxanthines, angiotensin-converting enzyme (ACE) inhibitors, and calcium channel blockers. The use of these may simply reflect the overlap of symptoms of BPD with asthma or the treatment of associated problems like pulmonary hypertension. The use of these agents solely as treatment for BPD (or to prevent development of BPD) has not been examined. For infants with pulmonary hypertension, nitric oxide (NO) may play an important role in promoting postnatal development of new blood vessels and may be critical for survival in patients with severe pulmonary hypertension. The use of the PDE5 inhibitor sildenafil or analogues, though often used for pulmonary hypertension, has not been well studied in the treatment of BPD but may play a role in prevention.43,44
Nutritional Support
Improved lung function and recovery from early lung injury requires adequate nutrition. For ex-premature infants to demonstrate adequate “catch-up” growth, they must eventually grow faster than average. It is unclear to what extent severity of lung disease will slow growth, but slower recovery and growth are directly correlated with smaller birth weights, later onset of enteral nutrition, and the presence of comorbidities.46,47
On average, an ex-premature will need to gain between 20 to 40 g per day to demonstrate catch-up growth; this period often follows a “lag” period of poor growth or “parallel” growth to the curve that is related to intercurrent illness or severity of lung disease. In general, catch-up growth starts as the disease’s overall severity lessens. 
 COMPLICATIONS
COMPLICATIONS
Complications are related to the therapy (ie, line infections, tracheitis) or to comorbid conditions that prevent the usual predicted improvement. In severe bronchopulmonary dysplasia (BPD), mortality rates as high as 20% to 40% have been reported.54 Progressive respiratory failure may develop and is often related to comorbid conditions such as aspiration, tracheo-esophageal fistula (TEF), gastroesophageal reflux disease (GERD), and so on, which may increase lung inflammation, increasing the risk of infection and death.
Tracheitis
Patients with tracheotomies frequently have recurrent clinical episodes of tracheitis.  For individuals with chronic or recurrent symptomatic tracheitis, antibiotics, including inhaled aminoglycosides, have been used to suppress or prevent such episodes. Such use has not been studied and remains empiric. Routine cultures of tracheal secretions can guide subsequent use of antibiotics, particularly in the patient prone to tracheitis. However, elimination of colonizing agents is usually impossible, so the use of repeated tracheal cultures to demonstrate airway sterility is pointless.
For individuals with chronic or recurrent symptomatic tracheitis, antibiotics, including inhaled aminoglycosides, have been used to suppress or prevent such episodes. Such use has not been studied and remains empiric. Routine cultures of tracheal secretions can guide subsequent use of antibiotics, particularly in the patient prone to tracheitis. However, elimination of colonizing agents is usually impossible, so the use of repeated tracheal cultures to demonstrate airway sterility is pointless.
BPD “Spells” and Exacerbations
BPD “spells” are acute cyanotic episodes of dyspnea more commonly seen with severe disease and patients on mechanical ventilation. The physiology is uncertain but may include bronchospasm or dynamic collapse of the airway. While bronchospasm may be promptly relieved by albuterol, a tendency for such spells to spontaneously resolve with hand-bagging or comforting often confounds understanding of what is helping the patient. If the patient has tracheobronchomalacia, beta-agonists may lead to paradoxic worsening,56,57 and sedation or even paralysis may be required to relieve asynchronous efforts with the mechanical ventilation. The mainstay of treatment remains oxygen therapy, comforting maneuvers, or sedation.
In contrast to brief spells, pulmonary exacerbations are more prolonged periods of worsening usually caused by a respiratory infection or sometimes by a too rapid weaning of support. Clinical findings may include wheezing or crackles, but these cannot be used to infer causation: beta-agonist, corticosteroid, anticholinergic, or diuretic therapy may still need to be tried empirically. If possible, cultures of blood and the tracheal secretions should guide the choice of antibiotic during such acute episodes of worsening. Prophylactic use of antibiotics does not reduce the incidence of pulmonary exacerbations and predisposes the patient to resistant organisms. Rarely, in life-threatening events, antiviral therapy can be used, based only on viral cultures.
In patients in whom suspected chronic airway inflammation is a contributing factor in recurrent exacerbations, initiation of inhaled corticosteroids can be considered. A failure to show gradual improvement, or a pattern of recurrent exacerbations, should always prompt a search for other comorbid conditions. Children with BPD should get all recommended age-appropriate immunizations.
Other Complications
Severe lung disease often leads to chronic air trapping with an increased anterior-posterior diameter of the chest. Infants who survive BPD are at higher than average risk for asthma, repeated hospitalizations, and neurodevelopmental abnormalities (see the “Prognosis or Outcomes” section below). Plagiocephaly is frequently seen in children with BPD, and a helmet designed to remodel skull shape is vital for children with severe BPD and associated delays.
 PROGNOSIS OR OUTCOMES
PROGNOSIS OR OUTCOMES
In managing the baby with bronchopulmonary dysplasia (BPD), it is important to keep in mind that even in survivors of a very traumatic early course, lung function will eventually improve. The lung health of patients with severe BPD has improved with the advent of surfactant therapy. As the disease evolves, the healing of damaged areas, and the recruitment of previously atelectatic areas, leads to improved ventilation-perfusion relationships and lung compliance. By the time patients are old enough to perform standard pulmonary function tests, their lung volumes and exercise capacity may be indistinguishable from nonaffected children.59-61 However, survivors do show evidence of impaired gas exchange and use different ventilatory strategies during exercise.62
 PREVENTION AND CONTROL
PREVENTION AND CONTROL
Advances in perinatal care have not eliminated BPD. Infants who previously would have died are now surviving, but often with chronic lung disease, whereas those who previously would have developed chronic lung disease now recover uneventfully. In a sense, then, BPD has been successfully prevented in many of those infants born between 28 and 34 weeks gestational age, who now rarely develop BPD compared to the presurfactant era. For the extremely low-birth-weight infant now surviving, the search for strategies to prevent BPD continues.
Preventing BPD requires identifying the factors that cause it and developing reasonable interventions. Two key areas of work have been in preventing preterm delivery and in improving perinatal care for those delivered early, particularly in ventilatory management. Factors associated with preterm delivery or low birth weights include socioeconomic status; race; obstetrical history; access to or utilization of health services; chronic inflammatory conditions, including periodontal disease, chronic chorioamnionitis, or Ureaplasma urealyticum infection; and younger or older maternal age.
When premature delivery is imminent, systemic corticosteroids provided to the mother prior to delivery have proven beneficial. Given the improved outcomes in premature infants from surfactant replacement therapy, ventilator strategies, and water-balance management, the relative value of antenatal corticosteroids may be decreased,71 while the potential for harm is increasingly recognized.
Ventilator strategies to prevent BPD have centered on avoiding the extremes that damage lungs. Permissive hypoxemia and permissive hypercapnia have been utilized to reduce oxygen toxicity and barotrauma, respectively. By “permitting” lower oxygen tensions or higher carbon dioxide tensions than are usual in normal respiration, lung injury can be avoided. While excessive oxygen can cause retinopathy of prematurity and lung damage, and inadequate oxygen can increase mortality, optimal levels of oxygen are still unknown.72
REFERENCES
See references on DVD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


