Breast Disease
Development, Anatomy, and Physiology
The first step in development of the embryonic breast is the appearance of a pair of longitudinal streaks, which are ectodermal thickenings on the ventral surface of the embryonic torso called the primitive mammary ridges, and commonly known as the ‘milk lines’.1 Paired lens-shaped placodes appear as thickenings at specific locations along these milk lines. These placodes may be numerous in other mammals, but generally develop as a single pair in humans. Epithelial cells invaginate from the placode into the underlying mesenchyme to form the breast bud. A dense mesenchymal stroma then coalesces around the bud. At the final stage of embryogenesis, proliferating epithelial cells sprout from the mesenchyme into a fat pad that has developed beneath the dermis.
There is a rudimentary ductal tree with lactiferous ducts established by 16 weeks. These ducts eventually coalesce at the developing nipple. The areola is seen first at 20 to 24 weeks, and a true nipple has developed by the final trimester. During the final weeks of gestation, placental estrogens stimulate the breast buds in both genders to enlarge and create a true breast nodule, about 1 cm in size, at birth. The ‘minipuberty of early infancy’, a bimodal surge in hypothalamic–pituitary–gonadal axis activity that occurs after birth, may lead to persistent breast enlargement well into the first few months of life.2 The fall of hormonal levels to baseline at around 6 months of age may stimulate prolactin secretion and the production of small amounts of milk (‘witch’s milk’). Throughout prepuberty, breast tissue is minimal and the nipple lies nearly flush with the skin in both boys and girls.
The onset of puberty is characterized by an increase in pulsatile secretion of gonadotropin-releasing hormone and gonadotropins that stimulate estrogen production by maturing ovarian follicles.3 The onset of development of a mature breast, known as thelarche, results from estrogen-stimulated ductal development and site-specific adipose deposition. In the absence of significant levels of circulating estrogen, male adolescents fail to produce a significant breast mass. This is not the case in females where the normal stages of the female breast development have been defined by Marshall and Tanner (Table 75-1).4
TABLE 75-1
Normal Stages of Breast Development in Females
| Stage | Description |
| Stage 1 | Preadolescent: elevation of papilla only |
| Stage 2 | Breast bud stage: elevation of breast and areola as a small mound, enlargement of areola diameter |
| Stage 3 | Further enlargement of breast and areola, with no separation of their contours |
| Stage 4 | Projection of areola and papilla to form a secondary mound above the level of the breast |
| Stage 5 | Mature stage: Projection of papilla only, resulting from recession of the areola to the general contour of the breast |
Data from Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969;44:291–303.
Pathophysiology
Benign female breast disease in children can be seen as an aberration of normal development and involution (ANDI).5 ANDI links adult breast pathology to events in early breast development. Thus, many of the same disease processes observed in adults may be present in children. Fibroadenoma is a disorder of normal lobular development. Excessive stroma development results in juvenile hypertrophy. Benign conditions that result from ductal involution seen in older adults may be encountered during infancy, such as periductal mastitis, nipple discharge, and nipple retraction.
Breast cancer is extremely rare in children. When it is encountered, it is almost exclusively seen in late adolescence.6 The study of early mammary development has importance because signals that control mammary gland embryogenesis and involution may be deregulated in breast cancer.7
Disorders of Development and Growth
Neonatal Hypertrophy
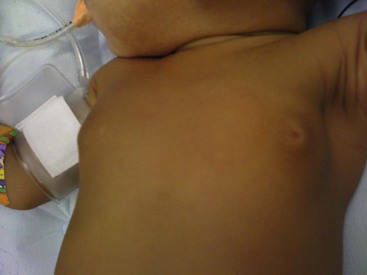
The newborn breast bud may enlarge in response to newborn prolactin that rises from falling levels of maternal estrogen in the days after birth (Fig. 75-1).8 These involute spontaneously within a few weeks without specific treatment.
Polythelia
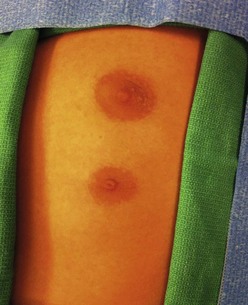
Extra nipples, areolae, and occasionally, a true accessory breast, may develop anywhere along the milk line from axilla to pubis in about 5% of children. They are most commonly located on the chest wall below the actual breast (Fig. 75-2).9 Unsightly polythelia should be excised.
Hypoplasia and Aplasia
Breast aplasia and hypoplasia may complicate Poland syndrome. Reconstruction of the chest wall and insertion of a breast prosthesis or breast reconstruction by a variety of flap techniques is usually indicated.10
Breast hypoplasia with an associated abnormality of pubertal development mandates an endocrinologic evaluation for ovarian failure, including gonadal dysgenesis, congenital adrenal hyperplasia, and varieties of disorders of sexual development.3 Breast augmentation is an option for these patients.
Incisions during a central venous catheter or chest tube insertion, as well as drainage of a breast abscess, may interfere with later breast growth and development.11 Extreme care must be taken when placing these incisions, particularly in prematurely born infants in whom the breast bud may be barely visible.
Atrophy
Atrophy of the breast may result from weight loss from any cause. Hypothalamic suppression and hypoestrogenism may complicate eating disorders, further retarding breast growth.12 In an otherwise well-nourished adolescent, breast atrophy should prompt a search for endocrine disorders that result in low estrogen or increased androgen.
Premature Thelarche
The differential diagnosis of breast enlargement in females is listed in Box 75-1. Breast development is defined as premature if it occurs before 6 years of age.13 Premature thelarche is defined as isolated breast development without findings of puberty, such as pubic hair, vaginal mucosal estrogenization, linear growth spurt, adult body odor, and pubertal behavioral changes. It is unilateral in 50% of cases, has a peak incidence between 6 months to 2 years, and resolves spontaneously in more than half of patients.
Premature thelarche can be distinguished from physiologic perinatal breast development and precocious puberty.3 Precocious puberty is associated with two or more of the other features of puberty previously listed, and has a peak incidence between 5 and 8 years later than true premature thelarche. Gentle retraction of the labia allows inspection of the vaginal mucosa, which is reddish and delicate during its prepubertal state, and pink and thicker when estrogenized. About 20% of girls with premature thelarche proceed to develop precocious puberty.
Drugs, toxins. and environmental causes of premature thelarche should be considered.14 A number of compounds have been implicated in the disorder, including xenoestrogens (compounds that bind to the estrogen receptor), phytoestrogens (compounds in plants), environmental toxins (pesticides, cosmetics, and packaging material), and estrogens in poultry, cosmetics, and hair products.
Hypertrophy
Virginal breast hypertrophy arises from exaggerated responses to pubertal hormonal fluxes. Both stroma and ducts are hypertrophic. Breast tissue and skin becomes ischemic and necrotic from the weight. Little experience is available with nonoperative treatment, although some improvement has been reported with tamoxifen.15 Reduction mammoplasty is an appropriate procedure for virginal hypertrophy.9 Continued breast growth may require repeated reductions.
Unilateral hypertrophy may produce breast asymmetry. Decisions regarding operation to correct the asymmetry require judgment as asymmetry is often seen between the two breasts. Ongoing breast growth may magnify the asymmetrical differences. Once Tanner stage 5 breast maturity is reached, equalization in the size of the breasts is reasonable with augmentation and reduction techniques.
Gynecomastia
Gynecomastia is the benign proliferation of glandular tissue of the male breast to the extent that it can be felt or seen as an enlarged breast (Fig. 75-3).16 Male breast enlargement can occur physiologically in the neonate, adolescent, or elderly. Gynecomastia that occurs before puberty warrants an urgent referral to a pediatric endocrinologist. Up to 60% of boys exhibit physiologic or pubertal gynecomastia. Although its etiology is not fully known, one hypothesis is that the effects of testosterone lag behind the estrogen effects at the onset of puberty. However, careful assays have not detected significant hormonal differences in boys with and without gynecomastia. Pubertal gynecomastia first appears between 10 and 12 years of age, with the highest prevalence at 13 to 14 years, corresponding to Tanner stage 3 or 4 (see Table 75-1). Involution is generally complete at 16 to 17 years, but may persist longer in obese boys.
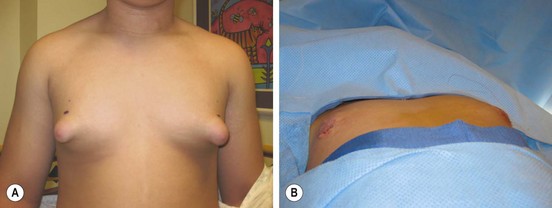
FIGURE 75-3 (A) This teenager has gynecomastia, which was causing him discomfort as well as having negative psychosocial ramifications. (B) On the operating table, the enlarged breast tissue was removed and a nice cosmetic appearance achieved.
Pathologic gynecomastia results from conditions that cause imbalanced estrogen and androgen concentrations. Elevated estrogen levels occur in neoplasms that secrete the hormone or its precursors (e.g., testicular germ cell, Sertoli cell, and Leydig cell tumors) as well as adrenal neoplasms. Feminizing adrenal tumors are generally malignant. Increased aromatase conversion of androgens into active estrogen occurs in obesity and in infants with hepatocellular carcinoma. Decreased androgen levels or androgenic effects can result from gonadal failure that may be primary (e.g., Klinefelter syndrome, mumps orchitis, castration) or secondary (hypothalamic and pituitary disease). Serum levels of sex-hormone binding globulin affect the balance of free testosterone and estrogen. Displacement of androgens from their receptors by the many drugs associated with gynecomastia (Table 75-2) may result in unopposed estrogen effects in sex hormone-sensitive tissue, including the breast.
TABLE 75-2
Drugs Associated with Gynecomastia
| Drug | Examples |
| Hormones | Androgens, anabolic steroids, estrogens, estrogen agonists and human chorionic gonadotropic |
| Antiandrogens/inhibitors of androgen synthesis | Bicalutamide, flutamide, nilutamide, cyproterone and gonadotropin-releasing hormone agonists (leuprolide and goserelin) |
| Antibiotics | Metronidazole, ketoconazole, β-minocycline, isoniazid |
| Anti-ulcer | Cimetidine, ranitidine, omeprazole |
| Abuse | Alcohol, heroin, amphetamines |
| Chemotherapy | Methotrexate, alkylating agents, Vinca alkaloids, cyclophosphamide |
| Cardiovascular | Digoxin, furosemide, spironolactone, angiotensin-converting enzyme inhibitors (captopril and enalapril), calcium channel blockers (diltiazem, nifedipine, verapamil), reserpine, amiodarone, α-methyldopa, sprionolactone, and minoxidil |
| Psychiatric/neurologic | Anxiolytic agents (e.g., diazepam), tricyclic antidepressants, phenothiazines, haloperidol, phenytoin, risperidone, clonidine, selective serotonin reuptake inhibitors |
| Other | Antiretroviral therapy for HIV, metoclopramide, penicillamine, phenytoin, sulindac, cyclosporine |
From Johnson RE, Murad MH. Gynecomastia: Pathophysiology, evaluation and management. Mayo Clin Proc 2009;84:1010–15. Reprinted with permission.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree