The preeminent risk factor for breast cancer is genetic inheritance of a mutation in the BRCA1 or BRCA2 gene.5,6 These are high-penetrance susceptibility genes located on the long arms of chromosomes 17 and 13, respectively.5,7 One mutated copy of either gene suffices to generate the hereditary breast and ovarian cancer syndrome, which is transmitted in an autosomal-dominant pattern.6 The proteins coded by the BRCA1 and BRCA2 genes are implicated in the homologous recombination repair of DNA double-strand breaks, an essential mechanism for maintenance of genome integrity.6,8 Besides predisposing patients to early-onset breast cancer and increasing risk of ovarian cancer, the hereditary breast and ovarian cancer syndrome also confers higher risk of developing pancreatic cancer, fallopian tube tumors, and uterine and cervical cancers.5,6
The prevalence of BRCA1/BRCA2 mutations in the general population is about 1 in 140 to 800 women, but it is higher in certain ethnic groups or populations, where founder mutations abound; among Ashkenazi Jews, for example, it is 1:40.5,8,9 The mean cumulative risk of developing breast cancer by age 70 has been estimated to be 57% for BRCA1 mutation carriers and 49% for BRCA2 mutation carriers; also, while mutation in either gene increases risk of gynecological cancers, BRCA1 mutation carriers tend to develop these tumors at a younger age than BRCA2 mutation carriers.5,6 Mutation in these genes also increases the likelihood of contralateral breast cancer, at a rate of 3% per year (Table 27-2).
Phenotypes of BRCA1 and BRCA2 Mutation-Associated Breast Cancers |
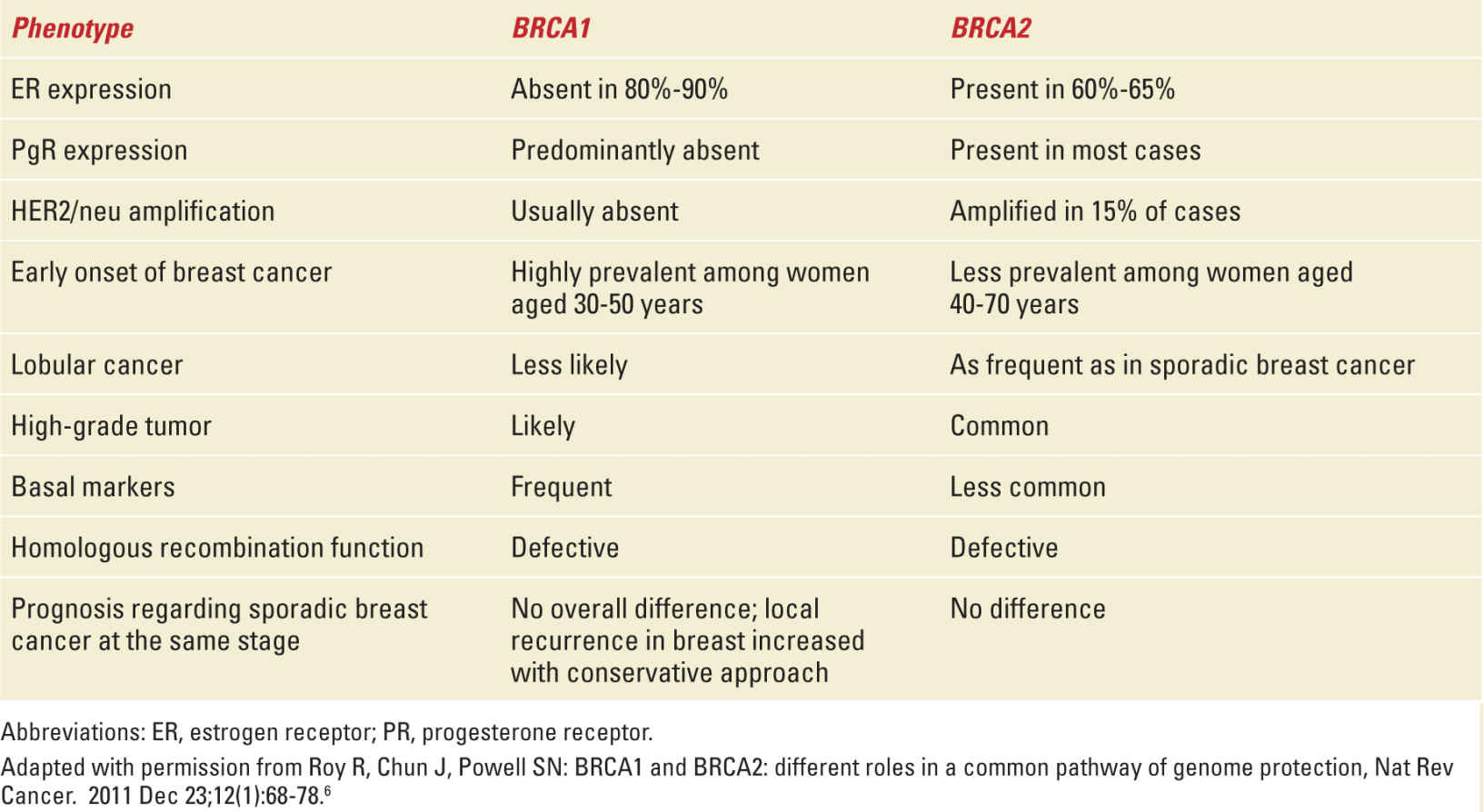
Approximately 2000 different mutations involving the BRCA1 and BRCA2 genes have been identified so far. Genetic testing can therefore be offered to patients and their families, always integrated in a genetic counseling approach, to assess risk and possible preventive/therapeutic strategies.5
ONCOFERTILITY IN THE FEMALE BREAST CANCER PATIENT
Oncofertility is a new interdisciplinary domain, a byproduct of the cross talk between the disciplines of oncology and reproductive medicine. The preservation of fertility for cancer patients diagnosed and treated during their childbearing years has proven to be a health-related quality-of-life issue of immense importance to the patient.10 This matter is even more pressing in breast cancer, because around 6% of female breast cancers in the developed world and 25% in the developing world are diagnosed in women who are younger than 40 years, and thus still in their reproductive years.11,12 Taking into account the current trend of postponing pregnancy to later in life,13 the expectation is that an increasing proportion of women will be diagnosed with cancer before completing their family.14
Oocytes suffer atresia and deteriorate in quality with advancing age. Cancer treatments may accelerate this process by not only destroying the ovarian reserve of oocytes (through blood vessel damage in ovaries, increased follicular apoptosis, and ovarian cortical fibrosis), but also affecting the entire hypothalamic–pituitary–gonadal axis.15,16 Local treatments for breast cancer—surgery and radiotherapy—do not have a substantial effect on female fertility, but systemic treatments such as chemotherapy, hormonal therapy, and targeted agents may.12
The net effect of chemotherapy on the female gonads is hard to define, as it depends on several factors: the type of chemotherapy, total dose, dose intensity, duration of treatment, and patient age and innate ovarian reserve.17,18 These last two are the most important host factors. Youth has a protective effect: the younger the woman, the more likely the ovary is to regain function after chemotherapy.11,12 Of all the chemotherapy drugs, alkylating agents, such as cyclophosphamide and cisplatin, have the greatest gonadotoxic potential (Table 27-3).12,19 It should be stressed that most of the research on gonadotoxicity of cancer treatments (chemotherapy and radiotherapy) has used amenorrhea as a surrogate marker of ovarian function, which seems to be incorrect,16,20 because under physiological circumstances the fertility potential declines years before the cessation of menses.16 On the other hand, other predictors of ovarian reserve, such as hormonal serologic testing (estradiol, anti-Müllerian hormone, inhibin B) and imaging (pelvic ultrasound for antral follicle count), still require long-term validation.16
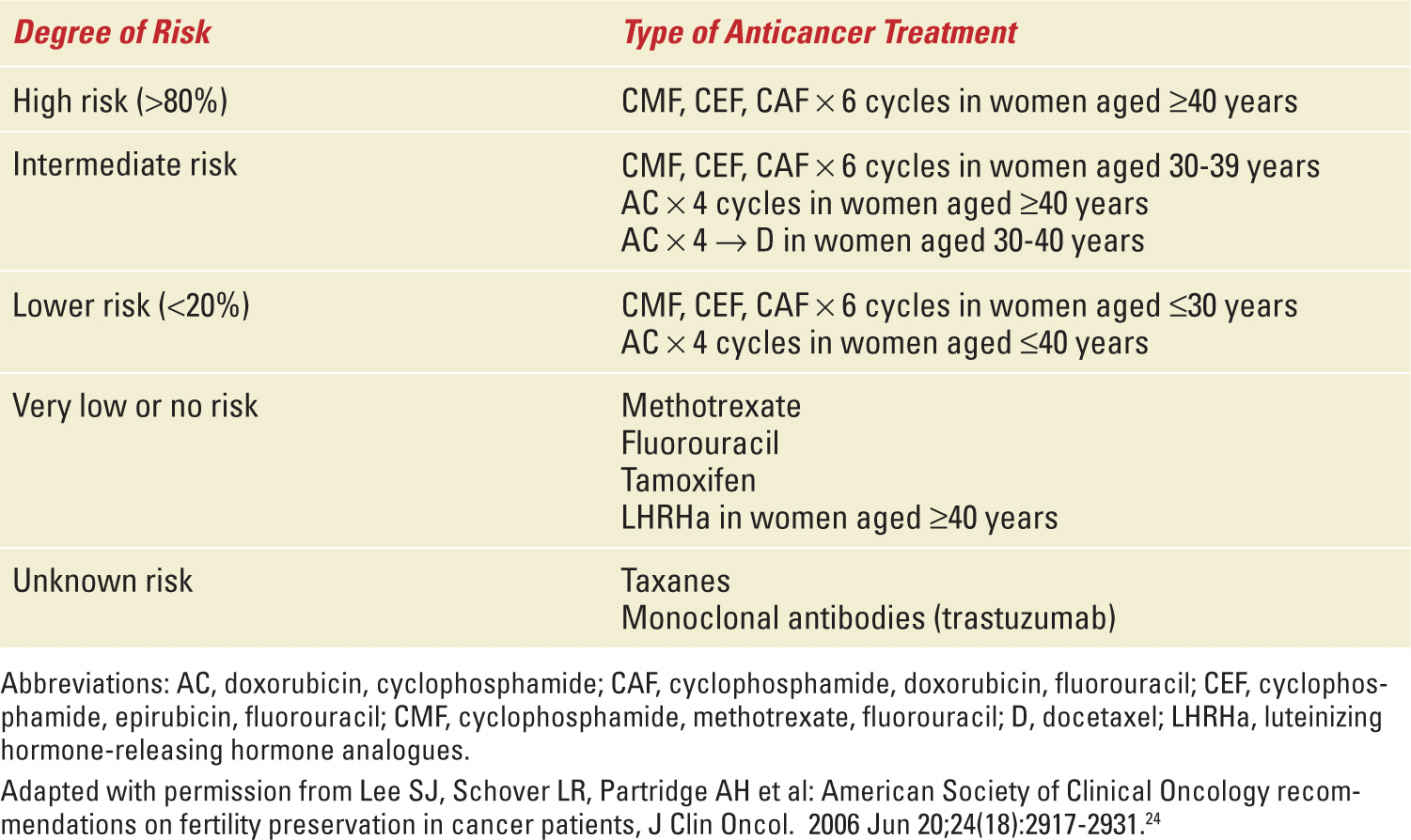
Risk of Permanent Amenorrhea Due to Anticancer Treatments in Breast Cancer Patients |
The prospect of a future full reproductive life is very important to female cancer survivors of reproductive age.21 The fertility preservation options are discussed in the subsequent section. Cancer patients typically prefer fertility preservation to other reproductive options such as donor gametes, adoption, and surrogacy. There are several reasons for this: (a) raising a child whose genetic background (and possibly early rearing, in cases of adoption) are unknown may entail parental insecurity22; (b) adoption agencies tend to identify cancer survivors as having a higher risk of premature illness or death than their noncancer counterparts throughout the life of the adoptee, who is their major concern by definition22; (c) cancer patients may attribute a value to the potential encompassed in ovarian tissue preservation and biogenetic parenthood as contributing to the concept of a normative life course already disrupted by the cancer experience22; and (d) cancer survivors usually prefer even a futile attempt at fertility preservation to not having the chance at all, because the lost opportunity is an irreversible event.22 Therefore, the possible loss of ovarian function due to anticancer treatments, with subsequent infertility and early menopause, is becoming a primary issue for young breast cancer patients, causing psychological distress and a negative impact on quality of life.21,23
Fertility Preservation Options
The American Society of Clinical Oncology (ASCO) and the European Society for Medical Oncology (ESMO) both recommend that health care providers make patients aware of the potential infertility problems as part of chemotherapy education and informed consent process before systemic cancer therapy is initiated. Fertility counseling should be offered to all young patients at risk of infertility in the face of upcoming cancer treatment.24,25
Key Issues in Fertility Counseling
According to the results of a recent survey of more than 1000 women on quality of life after cancer treatment, specialized counseling about reproductive issues is associated with less regret and greater quality of life, even though relatively few of the respondents had taken action to preserve fertility.26 On the other hand, patients whose treatments are not expected to cause gonadotoxicity may not need to be put through counseling.27
An interdisciplinary medical team comprising an oncologist, a reproductive endocrinologist, and a fertility gynecologist trained in fertility preservation techniques is needed to take care of female cancer patients facing fertility-threatening therapies.28 A critical step in providing cancer patients the appropriate information needed to make decisions about fertility preservation is referral to a reproduction specialist (typically a gynecologist). Oncologists with enough experience and knowledge in the field of fertility preservation may perform reproductive counseling themselves, only referring to a Reproductive Unit patients interested in cryopreservation strategies. Nevertheless, as was shown in the survey already mentioned, pretreatment fertility counseling by a fertility specialist and an oncologist resulted in significantly lower regret than counseling by an oncologist alone.26 Cooperation with one or more Reproductive Units is crucial for oncologists, to give their patients the opportunity to undergo well-timed and complete counseling.29,30 On the other hand, the Reproductive Unit must be able to manage these patients with minimal delay, offering the optimal option for each case.31 Therefore, a well-functioning relationship between the Oncology and Reproductive Units is the first step in management of fertility issues in cancer patients. Improving referral rates of cancer patients to fertility specialists begins with communication between oncologists and reproductive specialists.32
During fertility counseling, patients should be informed about the potential risk of ovarian function impairment (acute ovarian failure, infertility, and early menopause) due to anticancer treatments and the available strategies to preserve fertility, including the standard and experimental techniques, timing, possible complications, success rate, costs, and ethical implications. Fertility counseling should be tailored to the patient, because both the impact of anticancer treatments on ovarian function and the success rate of fertility preservation strategies are strongly linked both to the patient’s characteristics (particularly age and ovarian reserve) and to the treatment received.27
Effects of Anticancer Treatments on Gonadal Function
Anticancer treatments (radiation, cytotoxic chemotherapy, and endocrine therapy) may have a negative impact on gonadal function, inducing premature ovarian failure. Ovarian function may be affected permanently or temporarily. In general, the majority of breast cancer patients who are 40 years or younger recover menses within 1 year from cessation of treatment, while the incidence of posttreatment permanent amenorrhea is estimated at between 33% and 76% in breast cancer patients older than 40 years.11 However, even women who resume menses after anticancer treatment remain at increased risk of going through menopause at an earlier age than women who did not receive chemotherapy.33
The main factors in premature ovarian failure in breast cancer patients are patient age at diagnosis, type of chemotherapy regimen used, and administration of tamoxifen. Patient age is the most important factor; young patients, having a higher number of primordial oocytes, have a lower rate of premature ovarian failure. The incidence of therapy-related premature ovarian failure increases with age, being in the range of 22% to 61% in patients younger than 40 years and 61% to 97% in patients older than 40 years.34
The risk of therapy-related premature ovarian failure differs according to the dose, schedule, and type of cytotoxic regimen used. As might be expected, the total dose of chemotherapy directly relates to the rate of ovarian dysfunction.35 While alkylating agents (particularly cyclophosphamide) are the most gonadotoxic,36,37 the risk of treatment-related premature ovarian failure is low with methotrexate and fluorouracil,24 whereas that associated with anthracyclines (doxorubicin and epirubicin) is intermediate.31 Few data are available for taxanes. Fornier and colleagues reported a case series of 166 breast cancer patients younger than 40 years treated with adjuvant anthracycline and taxane-based chemotherapy; they showed that sequential administration of a taxane and an anthracycline-based regimen did not produce a higher rate of chemotherapy-related amenorrhea than the anthracycline regimen alone in historic control.38 In contrast, Okanami et al retrospectively compared the incidence of amenorrhea among 66 breast cancer patients treated with an anthracycline with or without a subsequent taxane; the addition of the taxane resulted in a higher rate of amenorrhea than the anthracycline alone.39 So far, the scarce data about the risk of amenorrhea with taxanes are still not conclusive. The incidence of chemotherapy-induced amenorrhea in breast cancer patients by type of regimen ranges from 9% to 93.9% (Table 27-4).38,40
Incidence of Chemotherapy-Induced Amenorrhea by Regimen Reported in Clinical Trials in Breast Cancer Patients |
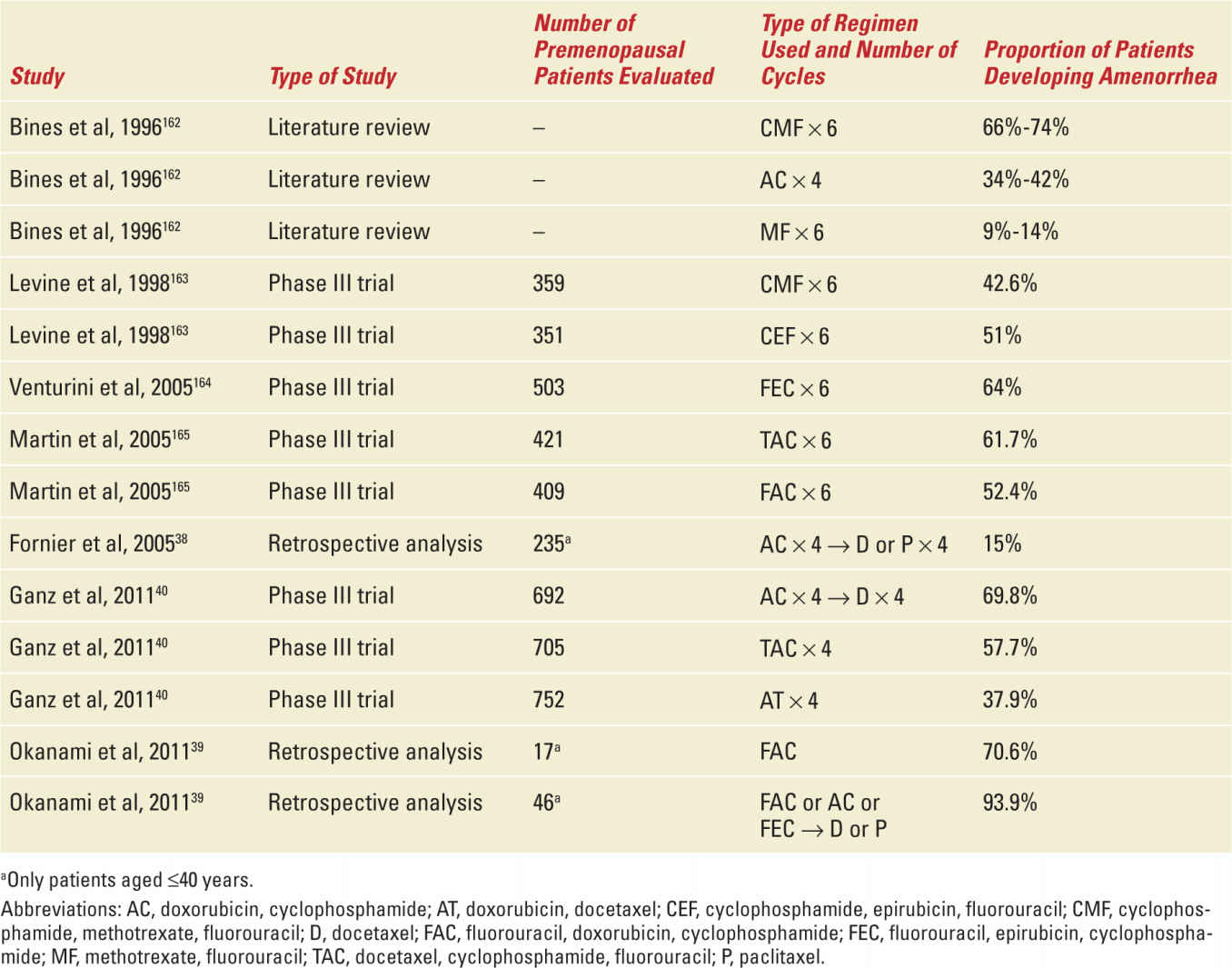
Of particular importance is the report by Ganz and colleagues, who studied the menstrual history and quality-of-life outcomes in breast cancer patients treated with adjuvant therapy within the National Surgical Adjuvant Breast and Bowel Project B-30 trial.40 In this trial, more than 5000 patients with early-stage, node-positive breast cancer were randomly assigned to one of three treatment arms: four cycles of doxorubicin and cyclophosphamide followed by four cycles of docetaxel (AC → T arm), four cycles of doxorubicin and docetaxel (AT arm), or four cycles of docetaxel, doxorubicin, and cyclophosphamide (TAC arm). A prespecified protocol analysis evaluated the rates of amenorrhea by treatment arm. The results showed a significant difference between treatment arms in the rates of prolonged amenorrhea at 12 months after the start of therapy: 69.8% for the AC → T arm, 57.7% for the TAC arm, and 37.9% for the AT arm (p <0.001). The amenorrhea rates were higher with the addition of tamoxifen. Approximately 61% of the women younger than 40 years experienced at least 24 months of amenorrhea, while that proportion neared 100% among patients older than 50 years. Patients in the AT arm reported significantly shorter duration of amenorrhea than those in the two other treatment arms.40 This study highlighted that, among chemotherapy agents, alkylating agents are associated with the greatest gonadotoxicity.
The ovarian components affected by chemotherapy-induced ovarian toxicity include the primordial oocytes, the granulosa cells, and the ovarian stroma. Two major mechanisms account for the ovarian toxicity due to chemotherapy: the direct induction of follicle and oocyte apoptosis41 and the vascular damage to the ovary.42 Oktem and Oktay developed a xenograft model to characterize the in vivo impact of chemotherapy agents on the human ovary; they showed apoptosis of primordial oocytes and growing follicle growths after 12 and 24 hours, respectively, and a 93% reduction of follicle density 48 hours after treatment.43 They then quantified the impact of chemotherapy on primordial follicle reserve and stromal function in the human ovary with a prospective controlled quantitative histologic and in vitro study, which showed significantly lower follicle counts in patients receiving chemotherapy than in untreated women.41 Among the chemotherapy group, women treated with a regimen that included an alkylating agent had significantly lower primordial follicle counts than those who received a regimen without an alkylating agent.41
A recent in vivo study analyzed the effect of cyclophosphamide administration on mouse ovaries, showing that its real mechanism for follicle loss is activation and growth initiation of the dormant primordial follicles.44 Furthermore, alteration of ovarian stromal function has been shown in patients previously exposed to chemotherapy, regardless of whether they were given an alkylating agent: ovarian cortex in these cases produced significantly less estradiol than that in controls.41 Additional processes that can lead to ovarian damage after chemotherapy are injury to blood vessels and focal damage to the ovarian cortex.42 The large cortical stromal blood vessels of ovaries previously exposed to chemotherapy showed prominent thickening and hyalinization of the wall, mild-to-moderate intimal fibrosis, and thickening of the muscular layer; these changes led to severe narrowing and obliteration of the vascular lumen. Furthermore, the cortex of ovaries exposed to chemotherapy showed proliferation of small blood vessels without any pattern of organization (“neovascularization”). Finally, ovaries exposed to chemotherapy revealed several areas of subcapsular cortical fibrosis with preservation of the ovarian surface epithelium.42
Among adjuvant endocrine therapies, tamoxifen as a single agent is associated with a relatively low risk of premature menopause, which is strictly dependent on age; in women older than 45 years, the risk of infertility with tamoxifen is 10% higher than that in controls.45 Nevertheless, the sequential administration of tamoxifen after chemotherapy is associated with a significant higher risk of infertility than chemotherapy alone.42,46 The luteinizing hormone-releasing hormone analogues (LHRHa) lead to ovarian suppression that is temporary; however, the reversibility of this effect is strongly influenced by patient age: the resumption of menses is expected in 90% of patients younger than 40 years but in 70% of women older than 40.45
In a study by Petrek et al, ovarian function was evaluated in 595 premenopausal breast cancer patients who underwent anticancer treatments; the surrogate of monthly bleeding was used to assess ovarian function. Significantly, different proportions of patients had monthly bleeding, depending on their age, chemotherapy regimen used, and time since chemotherapy administration. Particularly, the majority of women aged 40 years or older had no menstrual bleeding at the end of chemotherapy and no recovery of bleeding in the follow-up years, while 85% of women younger than 35 years had recovered menses at 6 months. The administration of a regimen containing doxorubicin and cyclophosphamide, with or without subsequent paclitaxel, dramatically decreased the proportion of women with monthly bleeding; after therapy, there was a slow recovery phase during which almost half of the patients continued to menstruate. On the other hand, a regimen containing cyclophosphamide, methotrexate, and fluorouracil resulted in a greater number of women with menses in the initial months after treatment, with a steadily diminishing proportion of patients with monthly bleeding at study end. Tamoxifen use decreased menstrual function between 12 and 24 months after chemotherapy.47
Assessment of Gonadal Function
The human ovaries have a fixed and nonrenewable number of primordial oocytes at birth, representing the so-called “ovarian reserve.”48 These oocytes cannot be regenerated and are progressively depleted during the reproductive life of every woman.49 Approximately 1,000,000 oocytes are present at birth, decreasing to 180,000 at menarche; this number continues to decline over time, with less than 1000 oocytes remaining at menopause.50 In addition to age, many factors can affect ovarian reserve, and thus consequently the success rate of various fertility preservation strategies and the expected damage induced by chemotherapy. Particularly, the most important risk factors that should be assessed through a proper clinical history are smoking habits, age at menarche, menstrual cycle duration, parity, family history of premature menopause, and existence of ovarian surgeries.51
In the past, menstrual cycling was considered the only marker to define recovery in ovarian function; however, its reliability is poor. In fact, estradiol level may remain high after chemotherapy-related amenorrhea and, vice versa, reproductive potential may be impaired in the presence of regular menses.52,53 Ovarian reserve can be evaluated quantitatively by antral follicle count with transvaginal ultrasound and hormonal markers such as follicle-stimulating hormone (FSH), inhibins A and B, and anti-Müllerian hormone (AMH) level.54 AMH level, while still being investigated for this application, is considered the most promising marker of chemotherapy-induced ovarian follicular depletion and an early plasma marker of chemotherapy-induced gonadal damage.55 AMH is a dimeric glycoprotein produced by granulosa cells from preantral and antral follicles, which reflect the ovarian follicular pool. Increased rates of chemotherapy-related amenorrhea have been associated with low pretreatment AMH and inhibin levels and high FSH levels.56 Moreover, AMH has been shown to have a potential role as an early indicator of chemotherapy-induced follicle loss57,58 and may become a tool for comparison of ovarian toxicity of different chemotherapy regimens.59 Unlike other hormonal markers, AMH levels are stable throughout the menstrual cycle; AMH level should be measured as soon as possible to make results available at the time of consultation.
Evaluation of the role of hormonal markers, particularly AMH, as predictors of the likelihood of chemotherapy-induced gonadotoxicity for application in tailored fertility preservation techniques awaits future studies.
Strategies for Fertility Preservation in Breast Cancer Patients
Four main fertility preservation strategies, standard and experimental, are available for breast cancer patients (Table 27-5): embryo and oocyte cryopreservation, ovarian tissue cryopreservation, ovarian suppression with LHRHa, and adoption and third-party reproduction. A patient’s options among these depends on several factors: her age and ovarian reserve, type of treatment received and its duration, availability of a partner at the time of diagnosis, available time before treatment initiation, and risk of metastasis in the ovaries.
Summary of Fertility Preservation Strategies Available for Breast Cancer Patients |
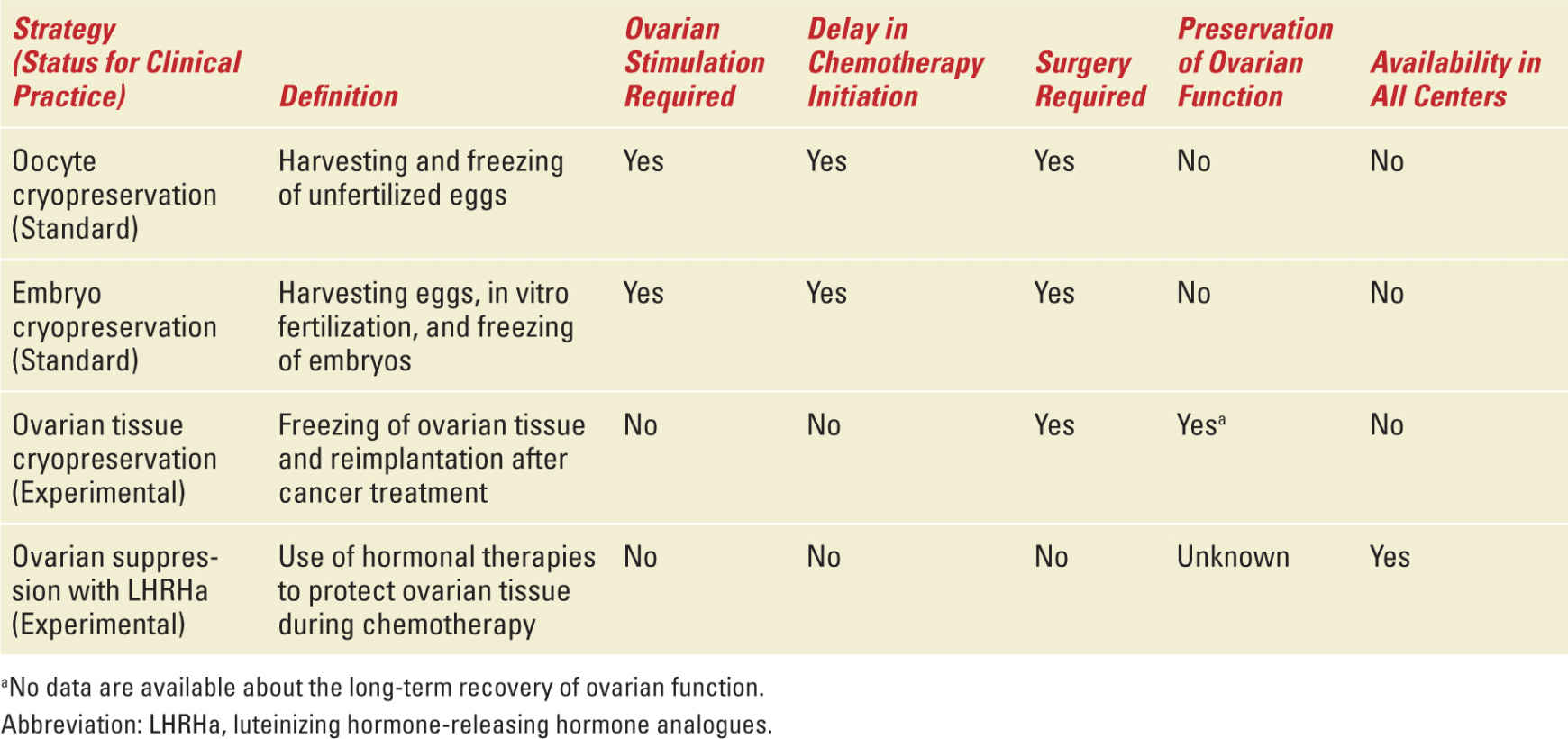
OOCYTE AND EMBRYO CRYOPRESERVATION—Oocyte and embryo cryopreservation are considered the standard fertility preservation option in cancer patients.60 Embryo cryopreservation has been the only established procedure for fertility preservation for many years; since January 2013, furthermore, oocyte cryopreservation is no longer considered experimental.61 Embryo cryopreservation is considered the standard strategy for patients with a partner at the time of diagnosis; oocyte cryopreservation is an alternative standard method, particularly for women without a partner. These techniques are not feasible in all breast cancer patients. The patients who are candidates for these strategies are those with a good ovarian reserve (generally, women not older than 38-40 years) and those who can delay chemotherapy initiation by 2 to 6 weeks. The controlled ovarian stimulation required to generate oocytes lasts about 9 to 15 days, and is usually started at the onset of the menstrual cycle.
These cryopreservation procedures require three different phases: (a) induction of multiple follicular growth through ovarian stimulation with daily injection of FSH from the onset of menses lasting about 9 to 15 days, requiring blood tests and serial ultrasound to monitor follicular development24; (b) oocyte collection performed by ultrasound-guided transvaginal needle aspiration with the patient under intravenous sedation (procedure lasting approximately 10 minutes)24; and (c) evaluation, selection, and subsequent cryopreservation of the oocytes collected. For embryo cryopreservation, the only difference is that the oocytes collected are fertilized in vitro through intracytoplasmic sperm injection and cryopreserved immediately after fertilization. So far, there are two available oocyte or embryo cryopreservation techniques: slow-rate freezing or vitrification.62,63 A recent meta-analysis showed the oocyte survival, fertilization, and optimal embryo quality rates were higher with vitrification methods than with slow freezing.64
There are two main safety issues for the application of these strategies in breast cancer patients: the need for controlled ovarian stimulation and the possible delay in chemotherapy initiation. Although the effect of a temporary increase in estradiol levels on the risk of disease recurrence is still uncertain, some concerns exist in cases of hormone-responsive tumors.65 To avoid the potential risk of short-term exposure to superphysiological estrogen levels, alternative approaches to ovarian stimulation by letrozole or tamoxifen have been proposed for breast cancer patients.66,67 As shown by Oktay et al, the concurrent administration of letrozole and gonadotropins during controlled ovarian stimulation led to estrogen levels similar to those in unstimulated cycles; the oocytes and embryos collected with this stimulation were comparable to those collected by standard ovarian stimulation protocols.68 The safety, in terms of recurrence and prognosis, of ovarian stimulation with concurrent administration of letrozole and gonadotropins before chemotherapy in breast cancer patients was evaluated by Azim et al in a prospective nonrandomized controlled study.69 Of 215 women with breast cancer counseled for fertility preservation, 79 underwent embryo or oocyte cryopreservation; at a median follow-up of 23.4 months after the end of chemotherapy, the hazard ratio for disease recurrence was 0.56 and the survival of patients who underwent controlled ovarian stimulation for in vitro fertilization procedures was not compromised compared with that of the 136 patients who underwent no fertility-preservation procedures (p = 0.36).69 However, long-term follow-up and further research are needed to confirm these findings.
The second concern is the possible delay in chemotherapy initiation. To overcome the need to wait for the onset of menses, “emerging protocols” with initiation of ovarian stimulation in the luteal or late follicular phases have been developed. Preliminary experiences showed promising results in terms of oocyte collection, with a significantly shorter time required to achieve oocyte or embryo cryopreservation.65,70,71
New techniques under development avoid both the exposure to estrogens during controlled ovarian stimulation and the delay in the start of anticancer treatment. These include cryopreservation of immature oocytes and in vitro maturation of oocytes. Immature oocytes can be collected without hormonal stimulation or with a short stimulation lasting 3 to 5 days; after collection, these immature oocytes are then matured in vitro before cryopreservation or cryopreserved at the immature stage and then thawed and matured in vitro before insemination.72,73 These techniques should be considered still experimental; so far, oocytes matured in vitro show significantly lower survival and fertilization rates than oocytes collected through the standard procedure.74
Few data are available on the pregnancy rate obtained with oocyte and embryo cryopreservation in cancer patients. Counseling patients about their likelihood of success should take into account data derived from the age-matched infertile population without cancer; in that group, the pregnancy rate after embryo cryopreservation is 19%, higher in women younger than 35 years.75 It has been suggested that stimulation and oocyte yields may be impaired in cancer patients even before they undergo anticancer treatments; as shown by a recent meta-analysis, the number of retrieved and mature oocytes collected was lower in untreated cancer patients than in noncancer controls (11.7 vs 13.5 total and 9 vs 10.8 mature, p = 0.003).76 Counseling regarding expected success rates may be difficult in cancer patients; more research efforts are needed.
CRYOPRESERVATION OF OVARIAN TISSUE—Cryopreservation of ovarian cortical tissue is a promising strategy but should be considered still experimental.25,60 The major advantages of this technique over oocyte and embryo cryopreservation are that it does not require any delay in the initiation of chemotherapy and it may save not only fertility but also the gonadal steroidogenic function. Worldwide, fewer than 30 pregnancies have been reported after transplantation of cryopreserved ovarian tissue, despite the large numbers of women who have undergone cryopreservation and subsequent reimplantation of ovarian tissue.77,78 The possibility of publication bias in reporting pregnancies after tissue cryopreservation and reimplantation should be considered; recently, authors called into doubt whether one of the reported live births resulted from the transplant or from the in situ ovary.79 In fact, the ovarian function generally remains unimpaired after the removal of ovarian tissue, making spontaneous pregnancies possible.80
This technique requires two surgeries: first, a laparoscopic or laparotomic surgery is needed to remove fragments of ovarian cortex, and then, once the patient has completed cancer treatment, the ovarian tissue is reimplanted.81,82 The ovarian tissue can be transplanted either to an orthotopic site (ovarian fossa, remaining ovary) or to a heterotopic site (eg, peritoneal abdominal wall, uterine serosa, or forearm).31 Ovarian tissue could be stored as whole ovary, fragments of ovarian cortex, or isolated follicles. Currently, the slow cooling method of cryopreservation is the most used in clinical practice; another technique under development, vitrification, has received considerable research interest.83 Ovarian function after reimplantation is expected to be restored in 3 to 6 months, but no data are available about the long-term recovery.84
The major concerns for application of the ovarian tissue reimplantation strategy are that two operations are required (at minimum), it is expensive, and there is a risk of reintroducing malignant cells when the tissue is reimplanted.85 So far, all ovarian tissue samples positive for malignant cells came from patients with hematologic disease, while no malignant cells have been found in ovarian tissue from breast cancer patients (by immunohistochemical analysis)86,87; accurate histological analysis of ovarian biopsy samples before reimplantation is mandatory.60
The efficacy of ovarian tissue reimplantation is difficult to determine because the number of cases is very limited compared with the number of patients who cryopreserved their ovarian tissue; the pregnancy rate per transplantation should be considered about 20% to 30%.88 The rate of ovarian functional recovery is high (90%-100%), but information on its duration is still limited to a few years.89
Ongoing research is investigating reliable methods for detecting residual disease in tissue grafts, better freezing-thawing methods, better revascularization techniques of the transplanted tissue, and optimal grafting sites.80,90
Few data are available about the number of patients who choose to undergo oocyte/embryo cryopreservation or ovarian tissue cryopreservation after fertility counseling; the percentage reported in the literature varies from 4% to over 50%.26,91,92 A better understanding of factors that influence patient choice is crucial to improve the quality of fertility counseling.
OVARIAN SUPPRESSION OF LHRH ANALOGUES—Pharmacological protection of the ovaries during chemotherapy is an attractive option to preserve fertility. The rationale for the use of LHRHa to reduce the ovarian toxicity of chemotherapy is that cytotoxic drugs mostly affect tissues with a rapid cellular turnover; the reasoning is that a state of induced gonadal quiescence during anticancer treatment may protect the gonads.93 It has been hypothesized that exploiting the inhibitory effect of LHRHa on ovarian function may reduce chemotherapy toxicity on the ovaries.94
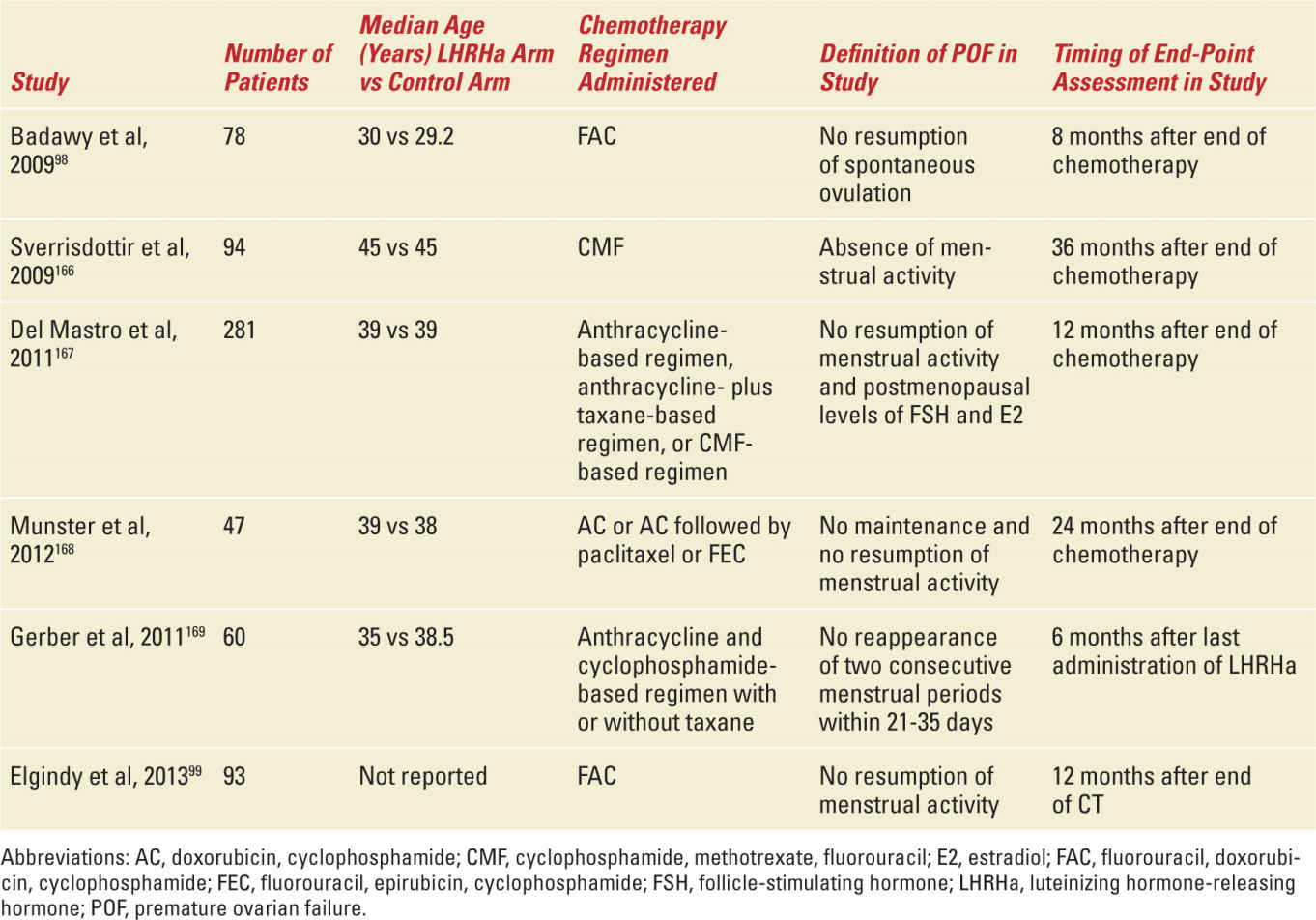
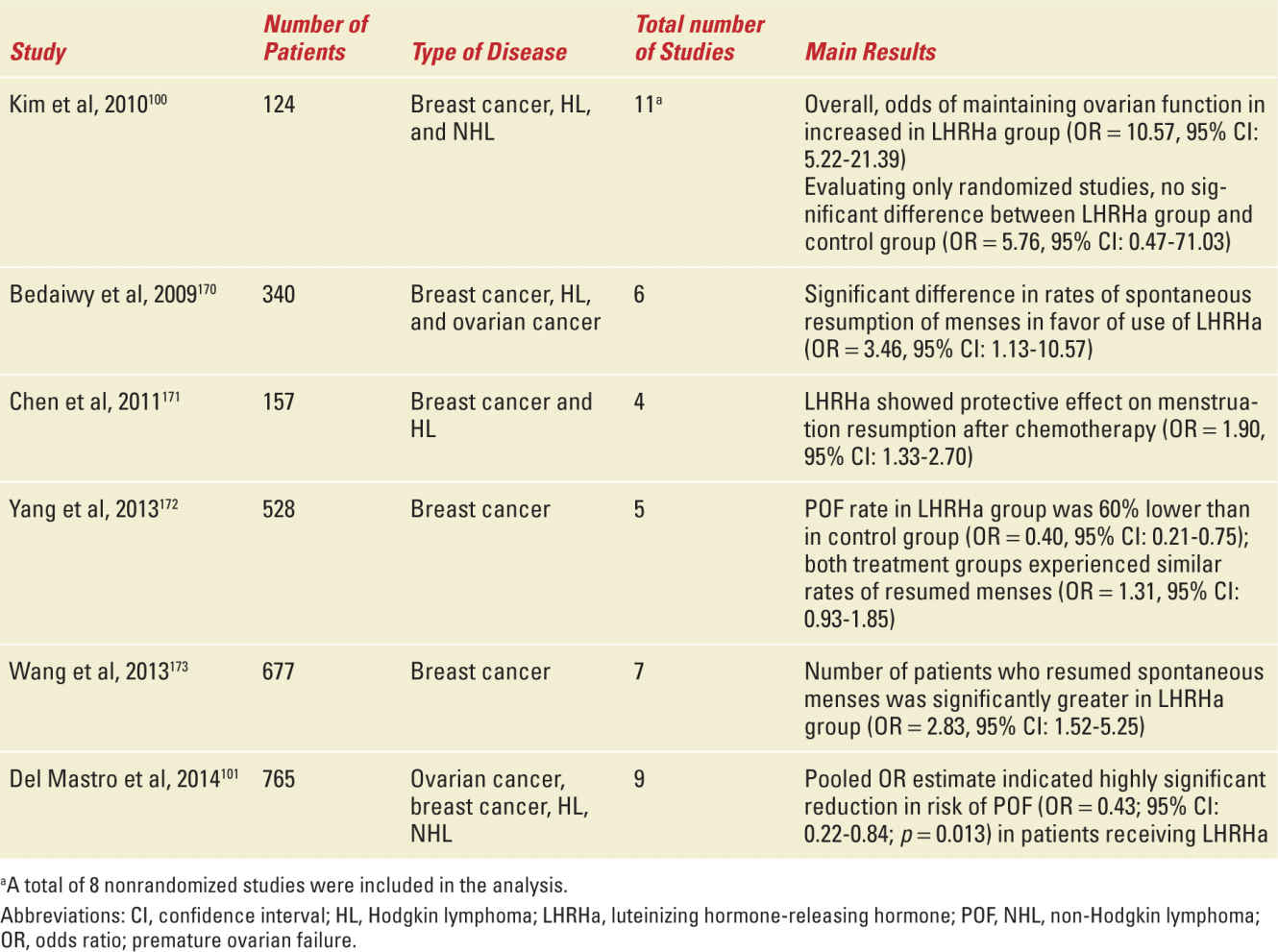
The effectiveness of the treatment was first reported in rats and monkeys.95,96 Several observational and phase II studies evaluated the potential protective effect of LHRHa on ovarian function during chemotherapy in breast cancer patients: the large majority (70%-100%) of women with breast cancer treated with LHRHa during chemotherapy did not experience premature ovarian failure.97 A total of six phase III studies evaluated the efficacy of LHRHa in preserving ovarian function in breast cancer patients who were candidates for chemotherapy (Table 27-6).98,99 In these studies, breast cancer patients were randomly assigned to receive adjuvant and/or neoadjuvant chemotherapy with or without LHRHa. Major limitations of these studies are the heterogeneous target populations, differences in selection criteria of patients for inclusion in the studies, differences in patient age at study entry, differences in chemotherapy regimens administered, variable duration of follow-up, and differences in the end points used to evaluate the efficacy of this strategy (see Table 27-6). A total of eight meta-analyses have been published analyzing the efficacy of this strategy; six analyzed the trials in breast cancer patients (Table 27-7).100,101 Overall, five of these six meta-analyses showed a significant reduction in the risk of premature ovarian failure in patients receiving LHRHa (see Table 27-7). Despite these extensive research efforts, the efficacy of LHRHa as a strategy to reduce the incidence of chemotherapy-induced premature ovarian failure in premenopausal women is still considered uncertain,102 and ovarian suppression with LHRHa is not considered a standard strategy to preserve fertility in ASCO and ESMO guidelines.25,60
Summary of Published Phase III Studies That Evaluated the Efficacy of Ovarian Suppression with LHRHa as a Strategy to Prevent Chemotherapy-Induced Premature Ovarian Failure in Breast Cancer Patients |
Published Meta-Analyses, Including Trials in Breast Cancer Patients, That Evaluated the Role of LHRHa in the Prevention of Chemotherapy-Induced Premature Ovarian Failure |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


