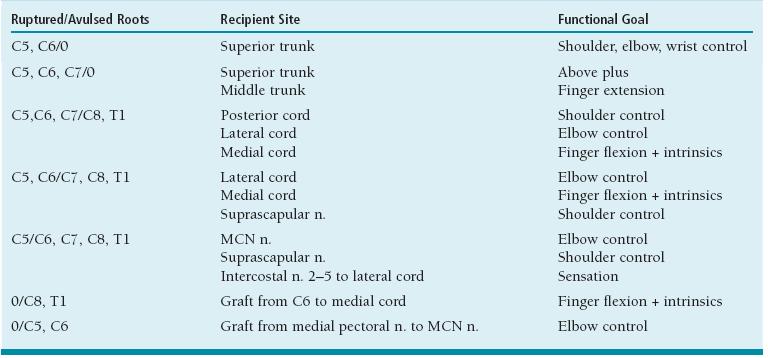
FIGURE 20-1 Normal anatomy of brachial plexus outlining nerve roots > trunks > cords > individual nerves. Obviously there are anatomic variations to this two-dimensional illustration, but this is the foundation of brachial plexus work.
Specifically, the typical patterns are that the (1) anterior divisions of the upper and middle trunks become the lateral cord; (2) the anterior division of the lower trunk becomes the medial cord; and (3) the posterior divisions of all three trunks become the posterior cord. The ulnar nerve is the terminal branch of the medial cord, the musculocutaneous (MCN) nerve of the lateral cord, the axillary and radial nerves of the posterior cord, and the median nerve from contributions of the medial and lateral cords. Along the way from nerve roots to terminal major nerves, the remaining muscles of the upper limb receive their neural supply from specific branches of the plexus. These branches are well outlined in Figure 20-1.
Any surgeon exploring and reconstructing the brachial plexus needs to be comfortable with the normal three-dimensional anatomy, facile in identifying anatomic variations, and able to do so in a distorted pathoanatomic situation. The brachial plexus lies between the anterior and the middle scalene muscles in the neck. The phrenic nerve arises from C3 to C5 (“3-4-5 keeps the diaphragm alive”) and courses on the anterior scalene muscle; the phrenic nerve is a key anatomic landmark and, once identified, may be traced proximally to the C5 nerve root and thus the brachial plexus.
A key surgical principle is to identify the neuroma and then healthy nerve roots proximal (if present) and nerves distal to the neuroma. Each nerve root is isolated as it exits the foramina. In the presence of avulsion injuries, the foramina will be empty. Distal to the neuroma, depending on the size of the neuroma and extent of injury, more normal individual divisions, cords, and/or nerves are isolated. Each situation is different and, accordingly, recognized, dissected, and recorded. The simple goal is to provide healthy neural input to affected muscles. The method by which this is achieved varies from case to case.
Etiology and Epidemiology
The incidence of BPBPs has been reported in 0.4 to almost 2:1,000 live births. The difference may relate to the type of prenatal and obstetrical care as well as average infantile birth weight and maternal pelvic size in different geographic regions. Identified perinatal risk factors include large gestational age infants (macrosomia, infants of diabetic mothers), multiparous pregnancies, previous pregnancies complicated by a birth palsy, prolonged labor, and difficult (shoulder dystocia) and assisted (vacuum, forceps) deliveries.1–6
The mechanism for injury as far as the nerve(s) is concerned is mechanical: a stretch or traction injury. This does not define who or what is responsible.7 The degree of force the nerve feels and the position of the arm and neck at the time of injury determine the extent of injury. The anatomic location of the injury is defined in each case. Usually, the neck and shoulder are distracted, and the upper plexus is injured. Thus the most common anatomic patterns for infantile indentlysis are upper trunk (C5–C6) followed by C5–C6–C7 palsies. Complete plexus palsies are less common, and isolated lower trunk palsies are almost reportable. The degree and type of neural injury is defined by the classic Seddon and/or Sunderland classification systems. The postganglionic injuries occur most often and are defined as neurapraxia, axonotmesis, or neurotmesis as opposed to preganglionic nerve root avulsions (Figure 20-2). The main issue is predicting the extent of spontaneous recovery: what will naturally recover and over what time frame. Neurapraxias fortunately are common and have full spontaneous recovery in the first weeks to months of life. The degree of axonotmesis defines the extent of natural history recovery, and this is more prolonged. Nerve recovery does not always follow classic anatomic pathways.8 The extent of natural recovery may not be known until 1 to 3 years of life.9 Some of these children may have better outcomes with surgery. Neurotmesis requires postganglionic reconstruction. Avulsion injuries have a poor prognosis by natural history and also tend to have a limited surgical outcome.10, 11
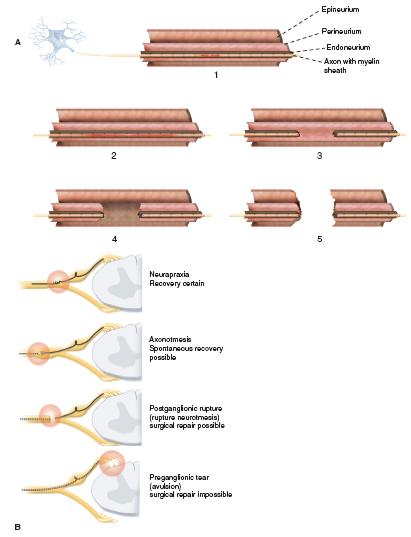
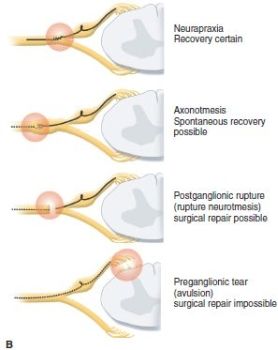
FIGURE 20-2 A: Sunderland classification of neural injury with neurapraxia (type 1 short segment and type 2 long segment neurapraxic injury), axonotmesis (type 3 disruption of nerve but perineurium and epineurium intact) while type 4 has disrupted perineurium and intact epineurium), and neurotmesis (type 5 with complete disruption of nerve). B: Sequence of nerve injuries from avulsion with no chance of recovery; to extraforaminal rupture with variable recovery (Sunderland type 3, and even 4); to nerve stretch (Sunderland types 1 and 2) with high likelihood of full recovery.
Clinical Evaluation
At birth, pseudoindentlysis from a clavicle or humerus fracture can be confused with true BPBP. Both conditions also can coexist. With a fracture, the initial and subsequent radiographs are diagnostic. Fractures in infants heal readily with palpable callus present almost always by 10 days to at most 3 weeks. The persistence of weakness beyond this time is concerning for a concomitant birth palsy. Other possibilities in the differential diagnosis besides fracture include congenital differences, central nervous system or spinal cord pathology, and infection.
Right from the start, the best information on the type of birth palsy is by physical exam. It is important to distinguish a preganglionic from a postganglionic lesion. Clear exam discriminators include a Horner syndrome (ptosis, miosis, enophthalmos, anhidrosis) and phrenic nerve palsy (elevated hemidiaphragm), which are associated with preganglionic injuries (Figure 20-3). Other concerning signs for avulsion include flail hand and loss of scapular control. The expectation in this situation is for very limited natural recovery since there is a complete disconnect between the brain–spinal cord axis and the peripheral nerve muscle axis at each avulsion level.
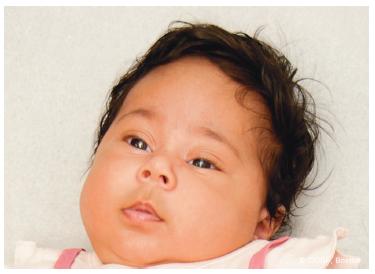
FIGURE 20-3 Horner syndrome with ptosis, miosis, enophthalmos, and anhidrosis. There is a high correlation between an avulsion injury and the presence of a Horner syndrome.
Sequential physical examinations are still the most reliable method to define the type and extent of extraforaminal injuries. Recovery is dynamic. The nerves will tell you how bad the injury is and the expected natural recovery if you examine the child frequently over the first 3 to 9 months of life.12,13 It takes patience and acquired skill to obtain an accurate exam. Utilizing more than one examiner for comparison and confirmation, such as a skilled plexus physical therapist, with each visit is beneficial. Observation of spontaneous movement, stimulated motor activity, and eliciting neonatal reflexes to assess muscle recovery are key components to an infantile exam (Figure 20-4). The systems most often used to classify these injuries are the Toronto test score (0 to 2 for elbow flexion; elbow wrist, finger, and thumb extension) (Figure 20-5); modified Mallet classification (grades I to V for abduction, external rotation, hand to mouth, hand to neck, and hand to spine); Hospital for Sick Children Active Movement Scale (0 to 7 grades for 15 upper limb movements); and Narakas (I to IV) (see Figures 21-4 and 21-5 in Chapter 21).14, 15 Invasive radiographic studies including myelograms, CT myelograms, and MRI scans (including fast spin echo, MRI myelograms, and MRI neurography) have been used to differentiate avulsions from extraforaminal lesions.16–18 These studies have high, but not perfect, accuracy. Electrodiagnostic studies are also utilized in some centers to define the severity of injury and prognosticate recovery. Unfortunately, there are multiple reports of discrepancies between electrodiagnostic and intraoperative findings. The neurophysiologic studies may underestimate the severity of injury and overestimate the prognosis of natural recovery.19 These studies always require conscious sedation, and some require general anesthesia. At present, most centers still rely on physical exam findings to advise parents on treatment options.

FIGURE 20-4 Typical posture of elbow extension (absent biceps), wrist flexion (absent wrist extensors), and digital flexion (absent finger extension).
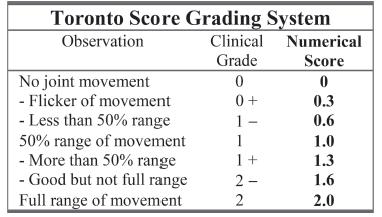
FIGURE 20-5 Toronto score for microsurgical indications. A score <3.5 is utilized in some centers as indicating nerve exploration and reconstruction. Others use the absence of biceps function as the indication for nerve surgery. Timing is debatable.
Surgical Indications
Always keep an open mind and a compassionate heart.
—Phil Jackson
Amazingly, after over 100 years of publications on nerve surgery in infants with BPBPs, the role, timing, and type of nerve surgery is still controversial.20–23 There is wide geographic variation. The timing controversy results in either potential unnecessary operations or missed opportunities to improve a child’s condition by surgery. Depending on when a surgeon chooses to intervene with microsurgery (at 3, 4, 5, 6, or 9 months of life),24–32 there is a significant difference in short-term economic cost and potentially long-term economic cost and outcome. We still do not have the definitive answers that can be applied to any given case. This ambiguity and lack of consensus complicates life for parents trying to decide what is best for their child’s future. The trends, however, are clear, and prospective study results are coming.
Inherent in any discussion of surgical indications is when not to do nerve surgery. Fortunately, the majority of BPBPs are transient. If partial antigravity upper trunk function including biceps function occurs in the first 6 weeks of life, ultimate normal recovery is expected. If biceps and upper trunk recovery does not begin by 3 months of life, then spontaneous recovery will be less complete. The crux of the ongoing debate centers on when between 3 and 9 months of life is the failure of biceps and upper trunk recovery an indication for infantile nerve surgery. Given the experience with secondary reconstruction procedures (e.g., tendon transfers), the question becomes which outcome will result in less long-term function: therapy and secondary transfers or nerve surgery and possible secondary transfers?
Clear indications for nerve reconstruction are (1) avulsion injury, (2) Toronto score <3.5 by 6 months of life,33 failure of return of upper trunk function as judged by partial biceps antigravity function by 6 months of life, and failure of “cookie test” by 9 months of life. There clearly is some overlap in these groups. Surgeon and institutional preference still abounds regarding surgery between 3 and 6 months of life. Our bias is for (1) avulsion surgery at 1 to 3 months, (2) extraforaminal rupture surgery at 5 to 6 months, and (3) rare “fell through the cracks” patients at 9 months. The last patients now usually have nerve transfers.
SURGICAL PROCEDURES
Champions keep playing until they get it right.
—Billie Jean King
The surgical options for nerve treatment are decompression, neurolysis, neuroma resection and nerve grafting, and nerve transfers. Simply, decompression and neurolysis are a part of the surgical exposure, evaluation of pathoanatomy, and intra-operative decision making. They are not part of definitive treatment, though almost everyone performs neurolysis of scarred-in nerves to assess fascicular continuity by microdissection and electrical stimulation. There is no evidence that decompression and neurolysis alone improve the patient’s future over natural history. Therefore, at present, your microsurgical options to hopefully improve your patient’s life as an independent adult are neuroma resection and grafting and nerve transfers.34–37 These same techniques are applied to the child and adolescent with a traumatic plexus injury. Direct repair to the spinal cord has not been feasible so far.38
As technically demanding as these operations are, the more complex aspect of the surgery is intraoperative decision making. There are treatment schemes that have been developed (Table 20.1) based on the available proximal donor and distal receipt sites, likelihood of neural recovery that will lead to functional muscle activity, and available secondary tendon transfers that will achieve satisfactory function. In general, recovery of shoulder stabilization, elbow flexion, and wrist extension are achievable goals in the more common extraforaminal ruptures in infants. Avulsion injuries are more challenging; but in infants, there usually is at least one nerve root to use as a proximal donor.
Table 20.1 Treatment recommendations in obstetric palsy
Adapted from Green, Hotchkiss, Pedersen, Wolfe, eds.: Green’s Operative Hand Survey, 5th edition, Philadelphia, Elsevier, 2005, 1333.
Also, in infants there is a reasonable chance of some recovery of hand function. However, even the most experienced surgeons are challenged when there is a mismatch between what is needed and what is available for reconstruction.
The tools available to aid intraoperative reconstructive decisions include (1) preoperative physical exam and MRI classification of injury, (2) microneural dissection to assess fascicular continuity, (3) intraoperative electrical stimulation of proximal nerves to assess distal muscle activation, (4) macroscopic and microscopic inspection of cut nerves, (5) biopsy of proximal nerve endings, and (6) somatosensoryevoked potentials (SSEPs). Motorevoked potentials have been too difficult to standardize and quantify for their effective use in decision making.
 Nonoperative Care of the Infant with BPBP
Nonoperative Care of the Infant with BPBP
At the time of birth, it is difficult to predict the future for the infant and parents (avulsion injuries excluded, which have a poor prognosis). In the past, there was probably an overabundance of reassurance regarding full recovery. Now, there may be heightened worry about permanent disability. Providing balanced, discriminating information for parents about their child is important.
Infants are usually assessed in the first month of life to establish a baseline exam and to develop a relationship with the parents. Many of these families come in prepared for an extensive discussion after research and communication with other affected families and caregivers. Serial exams on a 1- to 3-month basis in the first year of life allow for proper classification of each child’s injury and recovery. If and when these patients normalize their function, their care is discharged back to their primary care provider. If there is poor recovery, and microneural reconstruction is necessary, then trust and clear communication regarding expected outcomes has been established before extensive surgery is performed. Finally, in those who recover sufficiently not to warrant nerve surgery but who still have functional deficits, they are monitored with therapy until secondary surgeries may be appropriate. This can extend throughout their childhood with visits every 6 to 12 months.
Early therapy to maintain a full arc of joint motion is advised. This is particularly important for the glenohumeral joint. Muscle reinnervation is sequential and asymmetric; the adductors and internal rotators recover before the abductor and external rotators. The resultant muscle imbalance, if prolonged, can lead to contractures, joint deformity, and dislocation. There are similar concerns about contracture development with the hand. Early return of digital and wrist flexion before wrist and finger extension can lead to flexion contractures. Occupational therapy, including corrective splinting, is then beneficial. Finally, with biceps recovery, the children can develop an elbow flexion contracture if there is asymmetry between biceps and triceps strength or considerable cocontraction. This elbow flexion contracture may be addressed with therapy and at times splinting or serial casting.
 Nerve Grafting for Upper Trunk Lesion
Nerve Grafting for Upper Trunk Lesion
Failure of biceps, deltoid, and external rotation recovery alone or in combination with failure of wrist and finger extension by 5 to 6 months is a clear indication for nerve reconstruction. As mentioned in the indications section, this same clinical situation leads to microneural reconstruction at 3 or 4 months at other institutions (Figure 20-6).
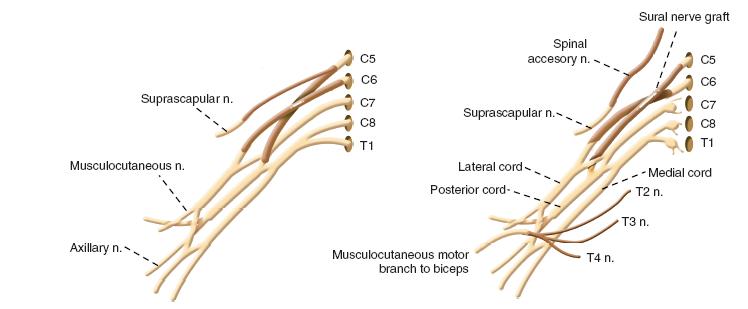
FIGURE 20-6 A: An illustration of a complete C5–C6 rupture with intact C7–T1. Reconstruction with cable sural nerve grafts from C5 to suprascapular and to posterior division of upper trunk; C6 to the anterior division of upper trunk. B: An illustration of C5-C6 grafting in combination with intercostal nerve (ICN) and spinal accessory nerve (SAN) transfers. C5 to posterior division of upper trunk; C6 to anterior division of upper trunk; SAN to suprascapular nerve; ICN to motor branch of musculocutaneous nerve to biceps.
Surgery is performed under general anesthesia without neuromuscular blockade. Both lower extremities are prepped in anticipation of nerve grafting, typically utilizing the sural nerve as donor nerves. Supine, modified beach chair position is used with a roll between the scapulae. The head is tilted toward the unaffected side, and the shoulder girdle, arm to fingers, and chest are all included in the operative field. This wide field allows for versatility of all nerve grafting and transfer options (Figure 20-7A). Exposure of the affected brachial plexus is through a transverse supraclavicular skin incision centered between the transverse processes of C5 and C6 (Figure 20-7B) (see Sidebar). After scoring the skin, a diluted epinephrine solution is injected in the incision to lessen bleeding. The platysma and supraclavicular fascia are divided in line with the skin incision. The supraclavicular cutaneous nerves and spinal accessory nerve (beneath the sternocleidomastoid muscle proximally) are isolated and protected. Crossing branches of the external jugular vein are ligated while protecting the longitudinal aspects of the vessel. The omohyoid muscle is tagged and divided. If necessary for exposure and reconstruction, the transverse cervical artery (which usually crosses at C7) and supra-scapular vessels are ligated and transected. The phrenic nerve is located on the anterior scalene muscle. Electrical stimulation while the anesthesia team momentarily holds the ventilation verifies diaphragmatic contracture. The nerve is followed proximally to identify C4 and C5. The more oblique C5 and C6 nerve roots are isolated between the anterior and middle scalene muscles. The upper trunk neuroma is usually at the junction of C5 and C6. Exposure and isolation of the more horizontal C7 is performed (Figure 20-8A). In each of these nerve root dissections, care is taken around the foramina to identify avulsions, assess neuromas in continuity, and protect against proximal bleeding. If there is any question about the viability of the C5 and C6 nerve roots for grafting, SSEP, transection with macroscopic and microscopic inspection, and biopsy are utilized. There is nothing worse than performing a major nerve reconstruction that fails to gain sufficient motor function due to the inadequacy of the proximal nerve segment. This is the critical intraoperative branching point between nerve grafting and transfers. The nerve roots are transected in sequence in preindenttion for grafting (Figure 20-8B, C).
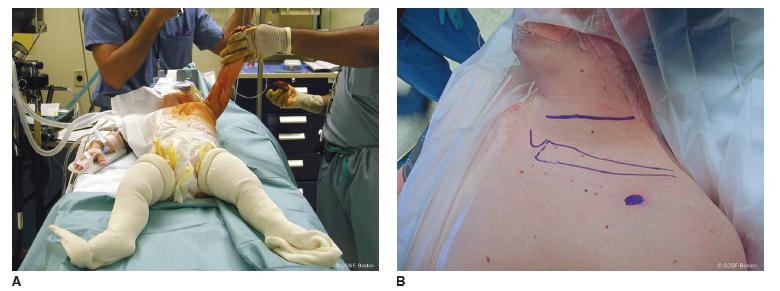
FIGURE 20-7 A: Prepping and draping for exposure of neck, shoulder girdle, and entire affected arm as well as bilateral sural donor grafts if necessary. B: Transverse incision for supraclavicular exposure of upper trunk rupture. The coracoid is outlined by blue dot. This can be modified with a curvilinear supraclavicular flap for more exposure.
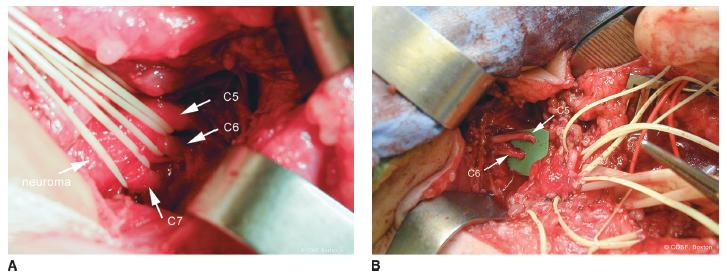
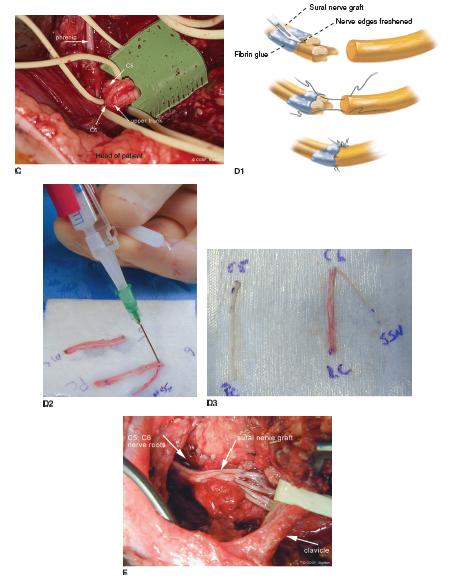
FIGURE 20-8 A: C5–C6–C7 nerve roots with elastic vessel loops around them as they enter neuroma involving upper and middle trunks. B: Transected C5 and C6 proximal to upper trunk junction in preindenttion for grafting.C: Close-up view of transected C5 and C6 nerve roots with healthy fascicles. The phrenic nerve is seen on the anterior scalene muscle as it arises from C5 and descends to the thorax. D: Use of sural nerve grafts for reconstruction, depicted schematically in D1. In this case, sural nerves were used to graft C5 to posterior cord and C6 to suprascapular nerve and lateral cord (D2-D3) E: Completion of grafting from C5–C6 and C7 nerve roots to suprascapular nerve, anterior and posterior divisions upper trunk, and posterior division middle trunk.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


