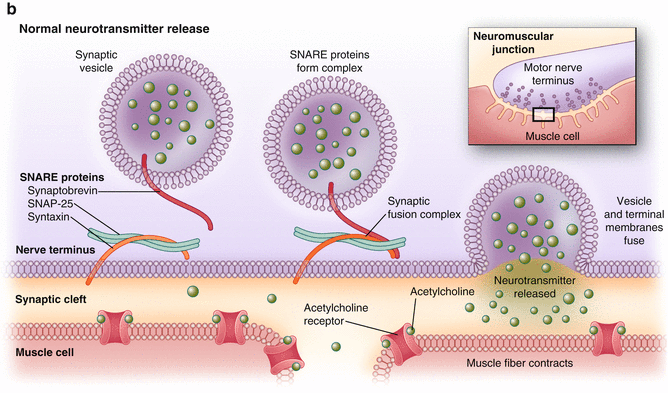
Fig. 17.1
(a, b) (a) Normal cholinergic transmission at the synaptic cleft. (Copyright © Allergan, Inc., Irvine, CA. Used with permission 2013.) (b) Onabotulinum toxin A works by inhibiting vesicle-mediated neurotransmission at the synaptic cleft

Fig. 17.2
Available doses of onabotulinum toxin A which are commonly used in urology applications (Botox, Copyright © Allergan, Inc., Irvine, CA. Used with permission 2013)
Use in Neurogenic Detrusor Overactivity
For years, patients with neurogenic bladder were limited to only a few options for treatment of their bladder complaints. Specifically, for the patient with neurogenic detrusor overactivity (NDO), options included watchful waiting, behavioral therapy, anticholinergic therapy, clean intermittent catheterization with anticholinergic therapy, or urinary diversion. The numerous side effects of anticholinergics include dry mouth, dry eyes, constipation, nausea, amongst others, which precludes their use in a great percentage of the population [5]. Because of these side effects, the majority of patients who begin anticholinergics will stop them at some point during therapy [6, 7]. With the success of onabotulinum toxin A injection into skeletal and smooth muscle in other areas, it would seem intuitive that it would provide a benefit in patients with NDO.
Patients with spinal cord injury (SCI) and multiple sclerosis (MS) often have NDO, which can lead to urinary incontinence, and has been shown to produce high storage pressures urodynamically [8]. Schurch and colleagues performed one of the first pilot studies evaluating onabotulinum toxin A for patients with neurogenic bladder due to spinal cord injury [9]. In this prospective, nonrandomized trial, patients who were on intermittent catheterization and had leakage despite anticholinergic therapy were given between 200 and 300 units of onabotulinum toxin A. Six weeks after injection, there was improvement in several urodynamic parameters, including maximum cystometric capacity (increase from 296.3 ± 145.2 mL to 480.5 ± 134.1 mL, p < 0.016), and in maximum detrusor voiding pressure (decrease from 65.6 ± 29.2 cmH2O water to 35 ± 32. 1 cmH2O, p < 0.016). In this study, the effect was noted to last approximately 9 months, when patients required repeat injection. This study also noted an increase in residual volume in treated patients, with residual volume on urodynamic evaluation increasing significantly from a mean of 261.8 ± 241.3 mL to 490.5 ± 204.8 (p < 0.016).
The United States Food and Drug Administration approved the drug for use in neurogenic detrusor overactivity in 2011. However, dosing and tolerability of the drug remained unclear. A phase-3 placebo-controlled study evaluated both the 200 and 300 unit dosage in patients with MS or SCI. This study included 416 patients from several centers around the world. The primary endpoint measure was the change in urinary incontinence episodes from baseline to week 6. In both the 200 and 300 unit treatment arms, mean weekly incontinent episodes decreased to a greater extent than placebo, 21 and 23 vs. 9 episodes per week, respectively (Fig. 17.3). This study had a high rate of postoperative urinary retention requiring intermittent catheterization, with 35 % of patients receiving 200 units, and 42 % of patients receiving 300 units, beginning catheterization. The definition of urinary retention was investigator-dependent, which, in this study, may have led to the somewhat high rates of clean intermittent catheterization in the early data [10].
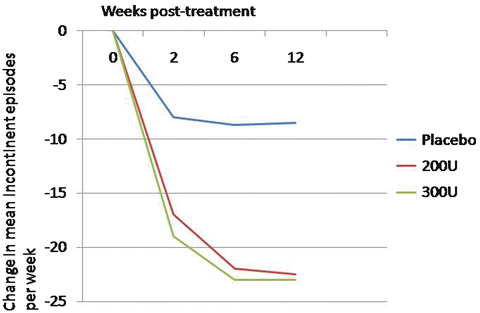

Fig. 17.3
Change from baseline in weekly incontinence episodes with 100 and 200 units of Botox compared to placebo in neurogenic patients (Adapted with permission from Ginsberg D, Gousse A, Keppenne V, Sievert KD, Thompson C, Lam W, Brin MF, Jenkins B, Haag-Molkenteller C. Phase 3 efficacy and tolerability study of onabotulinumtoxinA for urinary incontinence from neurogenic detrusor overactivity. J Urol. 2012 Jun;187(6):2131-9)
Use in Idiopathic Overactive Bladder
Approximately 16 % of the adult population has idiopathic overactive bladder (OAB), and approximately 1/3 of these patients have associated urinary incontinence [11, 12]. Patients with OAB are treated initially with behavioral therapy and pharmacologic therapy in the form of anticholinergics or beta 3-agonists. Like NDO, many patients with OAB who begin pharmacologic therapy with anticholinergics are unable to tolerate the side effects of dry mouth, constipation, and dry eyes, and therefore, the majority of patients stop these medicines within months of beginning treatment [13]. Onabotulinum toxin A represents a viable option for these patients, without the untoward side effects typically experienced by anticholinergic therapies.
Brubaker and colleagues compared 200 units of onabotulinum toxin A to placebo in a randomized trial involving female subjects [14]. These women were considered to have OAB with urge incontinence, with a minimum of six incontinent episodes over a 3 day period. Approximately 60 % of women receiving onabotulinum toxin A had a positive effect as evidenced on the Patient Global Impression of Improvement. The median duration of the response in these women was 373 days, compared to placebo, which was 62 days (p < 0.0001). This study was halted after 43 women were randomized, however, due to increased incidence of urinary tract infection and post-void residual volume in the women receiving onabotulinum toxin A.
Dmochowski published results of a phase 2, multicenter, randomized, double-blind study evaluating multiple dosing of onabotulinum toxin A [15]. Patients with 8 or more urinary urgency incontinence episodes per week and 8 or more micturitions per day were included in the study. Patients received 50, 100, 150, 200, or 300 units of onabotulinum toxin A or placebo. The primary endpoint in this study was urinary incontinent episodes at week 12 after treatment. Efficacy was noted in all groups treated with 100 units or greater of study drug. When dosage response curves were evaluated, it was evident that doses over 150 units did not provide any additional benefit.
Nitti and colleagues reported on the first phase 3 placebo-controlled trial evaluating the 100 unit dose in patients with refractory OAB. Patients with a minimum of three or more urgency incontinent episodes over a 3 day period and with eight or more voids per day were randomized to 100 units of onabotulinum toxin A or placebo. Not surprisingly, onabotulinum toxin A reduced daily incontinent episodes at a greater frequency than placebo (2.65 vs. 0.87 fewer episodes, p < 0.001) (Fig. 17.4). Total continence rates were 22.9 % in the onabotulinum toxin A group and 6.5 % in the placebo group. Additionally, nocturia, urgency episodes, and volume per void were improved in the study group versus placebo. Therefore, the US FDA approved the dose of 100 units of onabotulinum toxin A in patients with idiopathic OAB.
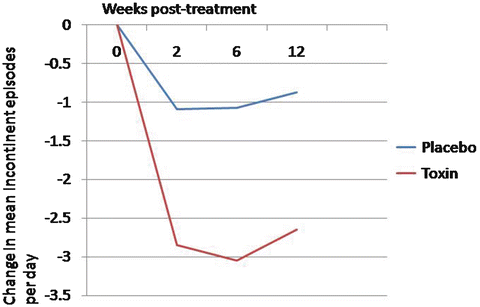

Fig. 17.4
Change from baseline in daily incontinence episodes with 100 units of Botox compared to placebo in patients with overactive bladder (Adapted with permission from Nitti VW, Dmochowski R, Herschorn S, Sand P, Thompson C, Nardo C, Yan X, Haag-Molkenteller C; EMBARK Study Group. OnabotulinumtoxinA for the Treatment of Patients with Overactive Bladder and Urinary Incontinence: Results of a Phase 3, Randomized, Placebo Controlled Trial. J Urol. 2013; 189(6):2186-2193)
There is no doubt that reducing urinary frequency and urge urinary incontinent episodes greatly improve quality of life in patients with overactive bladder. Sahai and colleagues demonstrated such a benefit in a randomized, placebo-controlled trial evaluating a 200 unit dose of onabotulinum A [16]. Overall, patients receiving onabotulinum toxin A had a significant improvement in their quality of life when compared with placebo beginning at the 4-week endpoint (median King’s Health Questionnaire Incontinence Impact domain score of 33 vs. 0, p = 0.03). This effect on quality of life improvement appeared to extend to 24 weeks in patients receiving onabotulinum toxin A through the open-label extension study.
There have been few direct comparisons of onabotulinum toxin A to anticholinergic therapy. One study randomized patients with idiopathic urgency urinary incontinence to receive daily solifenacin or trospium (5 mg solifenacin, with escalation to 10 mg, and if necessary, subsequent switch to trospium 60 mg extended release) plus an intradetrusor injection of saline versus intradetrusor injection of 100 units of onabotulinum toxin A plus oral placebo [17]. Two hundred forty-nine patients were randomized, and patients were evaluated at the 6-month point with voiding diaries and quality of life questionnaires. At 6 months, both groups had significant reduction in daily incontinence episodes (3.4 fewer in the anticholinergic group vs. 3.3 in the onabotulinum toxin A group, p = 0.81). Patients receiving anticholinergics were more likely to develop dry mouth versus onabotulinum toxin A (46 vs. 31 %, p = 0.02). Complete resolution of incontinence was noted more commonly in the onabotulinum toxin A group than placebo (27 % vs. 13 %, p = 0.003). Not surprisingly, patients in the onabotulinum toxin A group had higher rates of urinary tract infection (33 % vs. 13 %, p < 0.001) and need for catheter use (5 % vs. 0 %, p = 0.01).
Evaluation, Workup, Procedure, and Post-Procedure
Pre-Procedure Considerations
Typically, patients who have failed conservative therapies such as behavioral modification and anticholinergic or beta agonist therapy or patients who have contraindications to oral anticholinergic therapies are good candidates for injection therapy. Patients should be formally evaluated prior to undergoing an injection of onabotulinum toxin A. Patients presenting with refractory overactive bladder with symptoms of urinary urgency, frequency, with or without urge urinary incontinence should always be attempted on conservative therapy prior to considering procedural therapy for their condition. A formal history to ascertain that patients have failed conservative therapy such as behavioral modification and adequate oral therapy, including dietary history, should be ascertained when evaluating patients for onabotulinum toxin A injection. A 24–48 h voiding diary can often provide insight to voiding symptoms, and daily intake of caffeine and other fluids should be recorded. A basic urologic evaluation consisting of a detailed and proper pelvic exam, residual volume measurement, and urinalysis should be performed. Patients presenting with bacteriuria should be treated appropriately prior to considering injection. Pelvic floor muscle rehabilitation or biofeedback should be attempted as first-line therapy for voiding symptoms. Typically, patients should be tried on oral anticholinergics or beta-agonist therapy prior to being scheduled for onabotulinum toxin A injection.
Once patients have been selected to undergo detrusor injection of onabotulinum toxin A, they must be counseled specifically about the risks of injection, which are discussed elsewhere in this chapter. Because the risk of urinary retention exists, patients should consider learning intermittent catheterization prior to injection, should they experience difficulty when the toxin exerts its effect on the detrusor. It is recommended that patients are started on a flouroquinolone antibiotic or trimethoprim-sulfamethoxazole 1–3 days prior to the procedure. Patients should avoid concurrent aminoglycoside administration, as the effects of onabotulinum toxin A have been reported to be potentiated with concomitant aminoglycoside administration in prior studies [18]. Patients should discontinue antiplatelet agents or other anticoagulants prior to injection, if possible.
Surgical Procedure
Intradetrusor injection of onabotulinum toxin A is performed through a cystoscope, in either the office or ambulatory setting. Both rigid and flexible cystoscopes can be used, and there are a variety of needles which are available for injection. There are also rigid cystoscopes designed specifically for needle injection which are small and quite tolerable to the patient with only local anesthesia. Typically, needle gauges range from 21 to 25 gauge. When using needles through a flexible cystoscope, one must be careful not to damage the scope by threading the exposed needle tip through the scope. Therefore, a variety of sheaths and retractable needle tips are available for use during flexible cystoscopy (Fig. 17.5a–d).
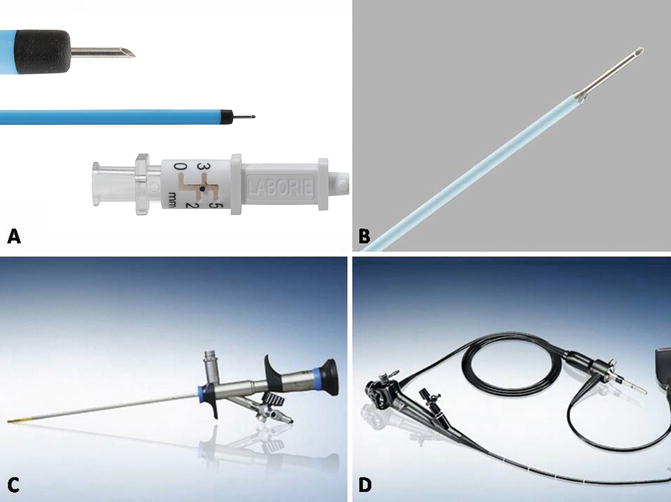

Fig. 17.5
(a–d) Commonly used instrumentation for injection. (a) InjeTAK needle. (b) Single-use flexible needle tip. (c) Rigid 14 French cystoscope. (d) Flexible cystoscope. (a: Courtesy of Laborie, Mississauga, Ontario; b, c, d: Courtesy of Olympus America, Inc, Center Valley, PA)
Anesthetic choice is surgeon-dependent, and patients should be offered no anesthesia, local anesthesia, intravenous sedation, or general anesthesia. Special considerations may be made in the patient presenting with NDO and a history of autonomic dysreflexia, as patients may require preoperative blockade to prevent unopposed sympathetic efferent discharge [19]. In the author’s experience, most healthy patients with idiopathic OAB tolerate injection in the office setting with simple local anesthetic. Such local anesthetic is in the form of 30–50 mL of 1 % lidocaine, which is instilled via catheter into the bladder. The solution is left for approximately 20 min in order to allow the anesthetic to exert its maximal effect. On the contrary, patients with multiple sclerosis, spinal cord injury with limited mobility may be better served under general anesthesia in order to allow for better positioning during the procedure. The patient is positioned in the lithotomy position, and genitalia should be prepped with sterile solution. The patient is draped, and anesthetic is administered if necessary.
Proper mixing onabotulinum toxin A is of utmost importance, as improper handling prior to the procedure may render the toxin ineffective. Onabotulinum toxin is stored in single-use 100 or 200 unit vials and is commercially available (BOTOX, Allergan Inc., Irvine, CA, USA). The vials themselves contain freeze-dried toxin which is in crystallized form, and is often not visible to the human eye. Botox is reconstituted by instilling 0.9 % normal saline into the vacuum-sealed vials. Total saline reconstituted depends on the total units being administered, in addition to anatomical and clinical considerations. One must remember not to shake the vial, as this can disrupt the delicate disulfide bonds within the toxin, rendering it ineffective. Therefore, careful rotation and mixing of the vial is all that is needed for proper reconstitution.
Cystoscopy is briefly performed to map patient anatomy, visualize the trigone and ureteral orifices, and to rule out any papillary mass or lesion. The bladder should be filled with at least 100 mL of sterile water or saline. The reconstituted drug is then injected at a depth of 2 mm, under direct vision at 20–30 equally spaced sites throughout the detrusor, sparing the trigone (Fig. 17.6). The trigone is extremely sensitive especially in patients with only local anesthesia. Furthermore, there exists the theoretical risk of new-onset vesicoureteral reflux if the trigone is injected. Sites should be spaced approximately 1 cm apart. A superficial bleb occurs if the injection is done superficially in the submucosa, whereas no visual change will occur if the drug is injected too deep. A proper injection should result in a subtle visual change and rise of the mucosa under the injection site. The author’s technique is to proceed from a left to right (or right to left) manner, beginning each column of injection sites at the bottom, to avoid any minor bleeding from a previous injection site seeping downward and preventing good visualization of the next injection site. For the final injection, 0.5–1 mL of sterile saline is injected in order to inject the remaining toxin, which remains within the needle sheath.
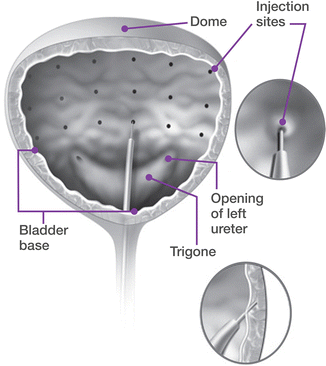

Fig. 17.6
Sites of injection for onabotulinum toxin A into the detrusor. The trigone should be spared, and sites spaced out equally to ensure uniform distribution of the toxin throughout the bladder (Copyright © Allergan, Inc., Irvine, CA. Used with permission 2013)
Post-procedure
In patients not already on intermittent catheterization, prior to being discharged after the injection procedure, patients should be able to void spontaneously. It is recommended that patients receive 1–3 days of antibiotics posttreatment in order to minimize the chance of urinary tract infection [Allergan PI]. Patients should be counseled that urinary tract infection is common, and they may have dysuria, or hematuria, as with any cystoscopic procedure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


