Bleeding Disorders
INTRODUCTION
This chapter reviews common neonatal bleeding disorders. These disorders can present challenges in diagnosis and management. Most neonatal bleeding defects are acquired; however, some inherited hemorrhagic disorders can present in the neonatal period.
Understanding the normal hemostatic process and the developmental aspects of the neonatal hemostatic system is important when investigating a neonate with a suspected bleeding disorder. The physiology of hemostasis in newborn infants and the approach to evaluation of neonatal bleeding disorders are discussed in the following introductory sections.
Overview of Normal Hemostasis
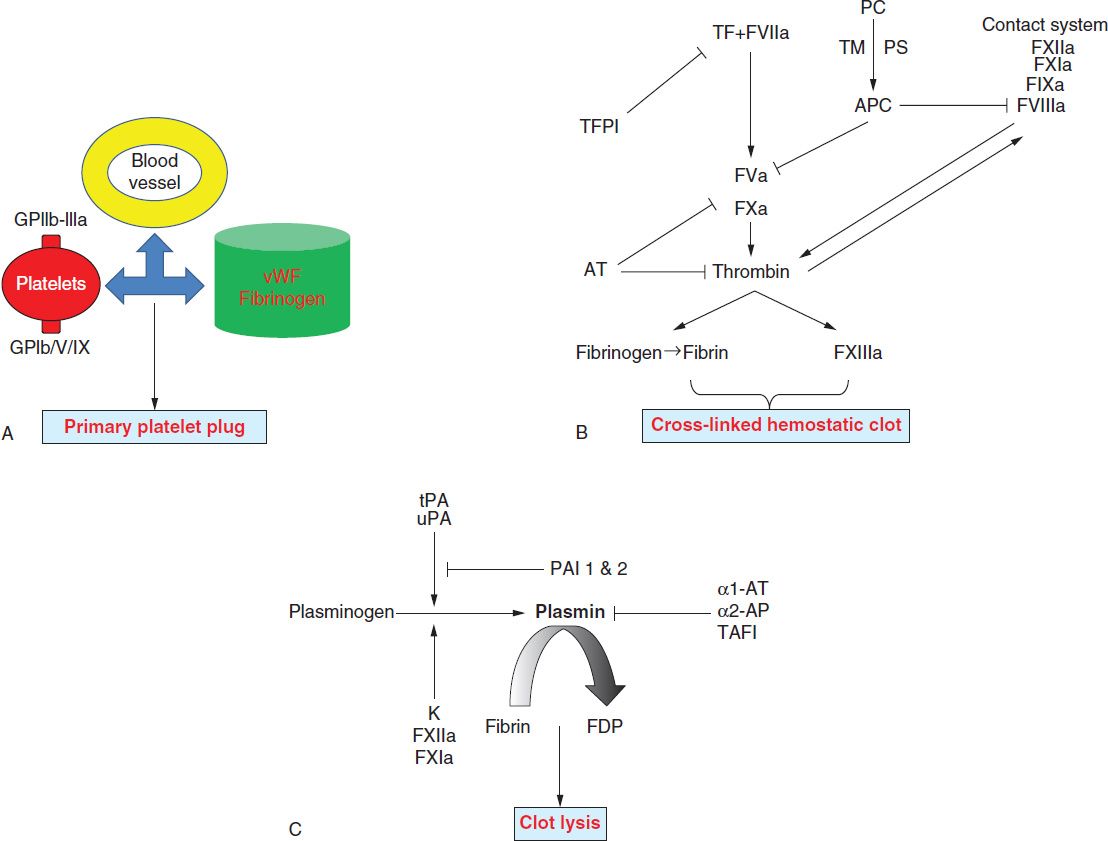
The key elements of the hemostatic system include the vascular endothelium, platelets, and coagulation. The immediate response to vascular injury is transient arteriolar vasoconstriction due to reflex neurogenic mechanisms and local secretion of vasoactive factors. This is followed by activation of platelets and coagulation proteins. Finally, once bleeding is controlled, blood vessel patency is restored by the fibrinolytic system. Hence, the normal hemostatic response can be viewed to occur in the following three phases (Figure 33-1):
FIGURE 33-1 Overview of the three phases of hemostasis. A, Primary hemostasis is dependent on interactions between platelets, the vascular endothelium, and coagulation proteins (von Willebrand factor [vWF] and fibrinogen). vWF associated with collagen in the exposed subendothelial matrix interacts with platelet GpIb/V/IX complex to mediate “platelet adhesion,” while vWF and fibrinogen bind platelet GpIIb-IIIa to mediate “platelet aggregation.” B, Secondary hemostasis involves sequential activation of coagulation factors. Formation of factor VIIa-tissue factor (TF) complex is the major initiating event that results in the generation of small amounts of thrombin (initiation phase or intrinsic pathway). These amounts of thrombin activate platelets and additional coagulation factors (amplification phase or extrinsic pathway), which results in a burst of thrombin formation, so that a stable fibrin clot can be formed. This process is subsequently terminated by three types of natural anticoagulants: antithrombin (AT), which inhibits the activity of thrombin and factors IXa, Xa, XIa, and XIIa; protein C (PC), which is activated by thrombomodulin (TM), thrombin, and its cofactor protein S (PS), inhibits factors Va and VIIIa; and TF pathway inhibitor (TFPI), which inhibits factor Xa and TF/factor VIIa. C, Tertiary hemostasis (fibrinolysis) functions to remove formed clots after hemostasis is secured. α1-AT 1 and 2, α1-antitrypsin 1 and 2, respectively; F, factor; FDP, fibrin degradation products; PAI 1 and 2, plasminogen activator inhibitor 1 and 2, respectively; tPA, tissue plasminogen activator; uPA, urokinase plasminogen activator.
1. Initiation and formation of the platelet plug (primary hemostasis)
2. Propagation of the clotting process by the coagulation cascade followed by termination of clotting by antithrombotic control mechanisms (secondary hemostasis)
3. Removal of the clot by the fibrinolytic system (tertiary hemostasis)
Vitamin K Physiology in Neonates
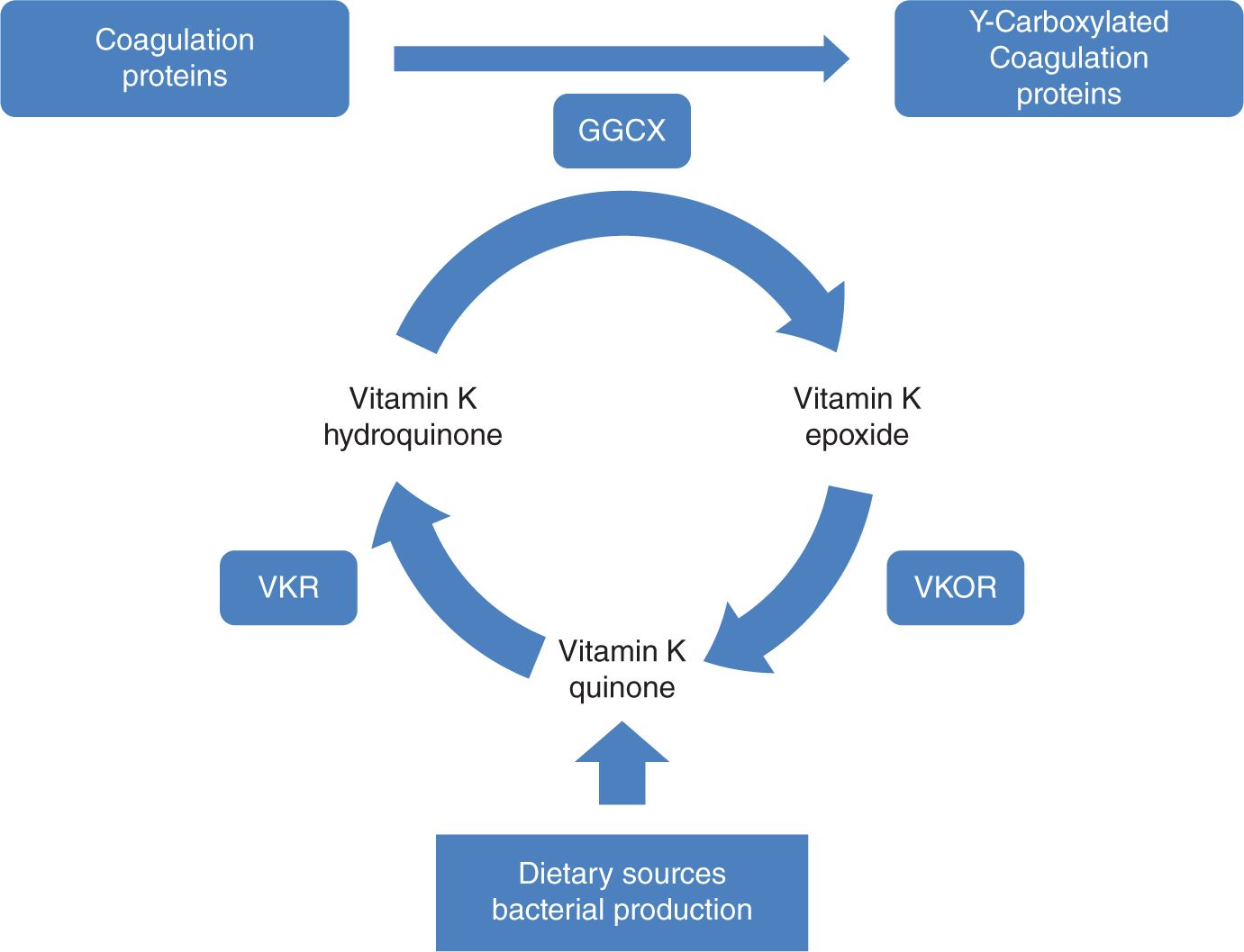
Vitamin K is present in a variety of dietary sources and is produced by intestinal bacteria. Vitamin K is a cofactor for γ-glutamyl carboxylase, an enzyme that performs posttranslational carboxylation, converting glutamate residues in proteins to γ-carboxyglutamate residues (Figure 33-2). These residues facilitate membrane interactions mediated by calcium ions and are necessary for proper function of the coagulation factors II, VII, IX, and X and proteins C and S. The newborn infant has a particularly fragile vitamin K status because of (1) limited hepatic stores at birth; (2) limited placental transfer of vitamin K; (3) variable and limited content of vitamin K in breast milk; and (4) initially, a sterile gastrointestinal system.1
FIGURE 33-2 The vitamin K cycle. GGCX, vitamin K f-glutamyl carboxylase; VKR, vitamin K reductase; VKOR, vitamin K epoxide reductase.
Developmental Hemostasis in the Neonate
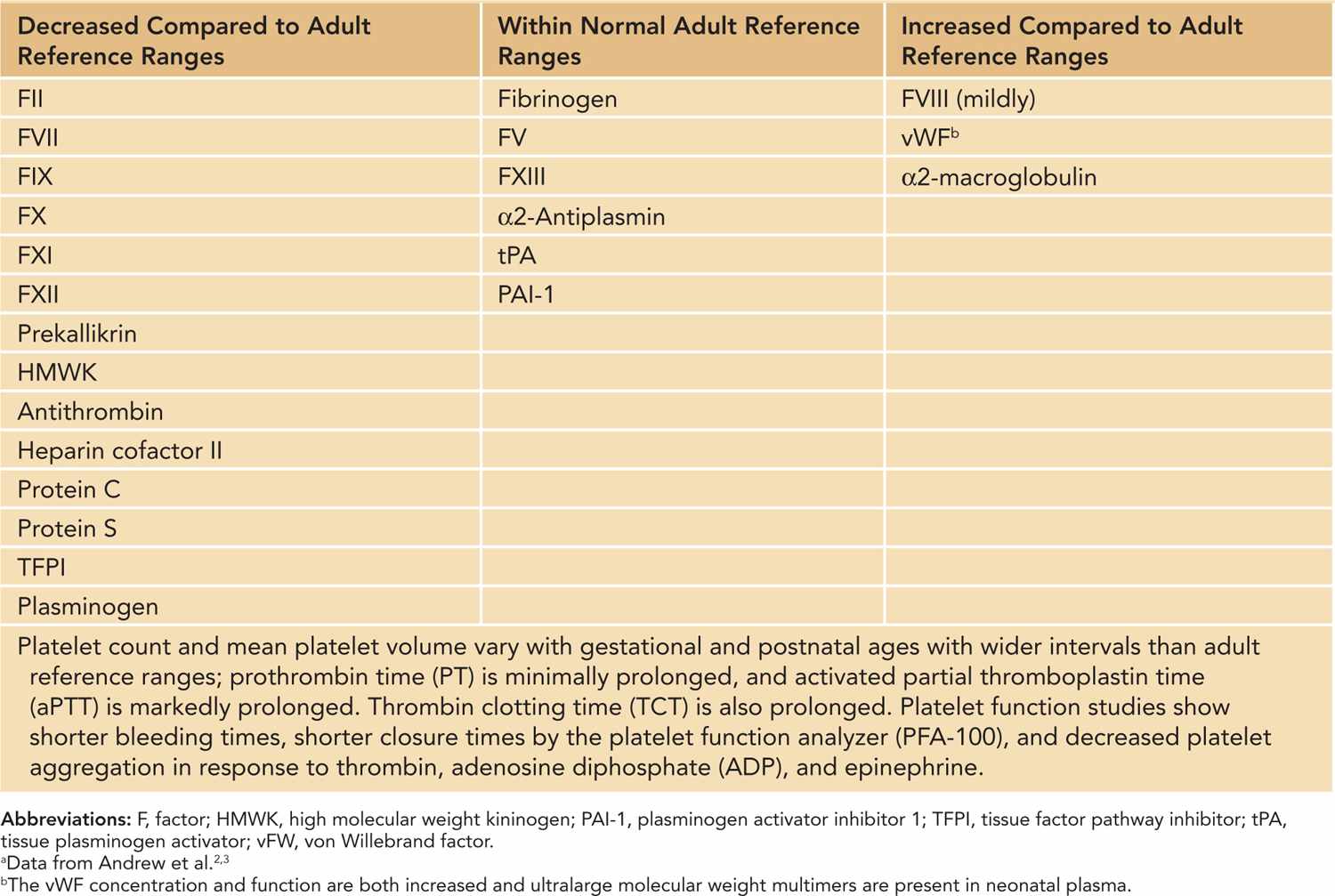
Proteins required for hemostasis do not cross the placenta and are synthesized by the fetus. Distinctive, quantitative, and qualitative gestational-age-dependent differences involving various components of hemostasis have been well characterized in neonates2,3 (Table 33-1). These differences are more pronounced in premature infants. Although neonatal platelets are hyporeactive, the concentration and function of von Willebrand factor (vWF) are increased in neonates due to the presence of ultralarge, more functionally potent vWF multimers.4 Vitamin K–dependent factors II, VII, IX, and X and contact factors XI and XII are reduced to about 50% of normal adult values, while the levels of the factors V, VIII, and XIII are similar to adult ranges. Although fibrinogen concentration is within normal adult ranges, it exists in a “fetal” form in the first 3 weeks of life. Fetal fibrinogen has a different composition compared to adult fibrinogen (increased sialic acid and phosphorous content) and shows a decreased rate of fibrin polymerization, which results in prolonged thrombin clotting time (TCT) in normal neonates.5 Similarly, concentrations of antithrombin, protein C, and protein S are significantly lower at birth, and the overall fibrinolytic capacity is decreased in neonates. As a result of these counterbalanced differences, the neonatal hemostatic system remains physiologically intact but lacks adequate reserve under stress conditions. Therefore, the risk of bleeding is increased in sick neonates and is further increased in premature infants.
Table 33-1 Hemostatic Parameters in the Newborna
Approach to Neonatal Bleeding Disorders
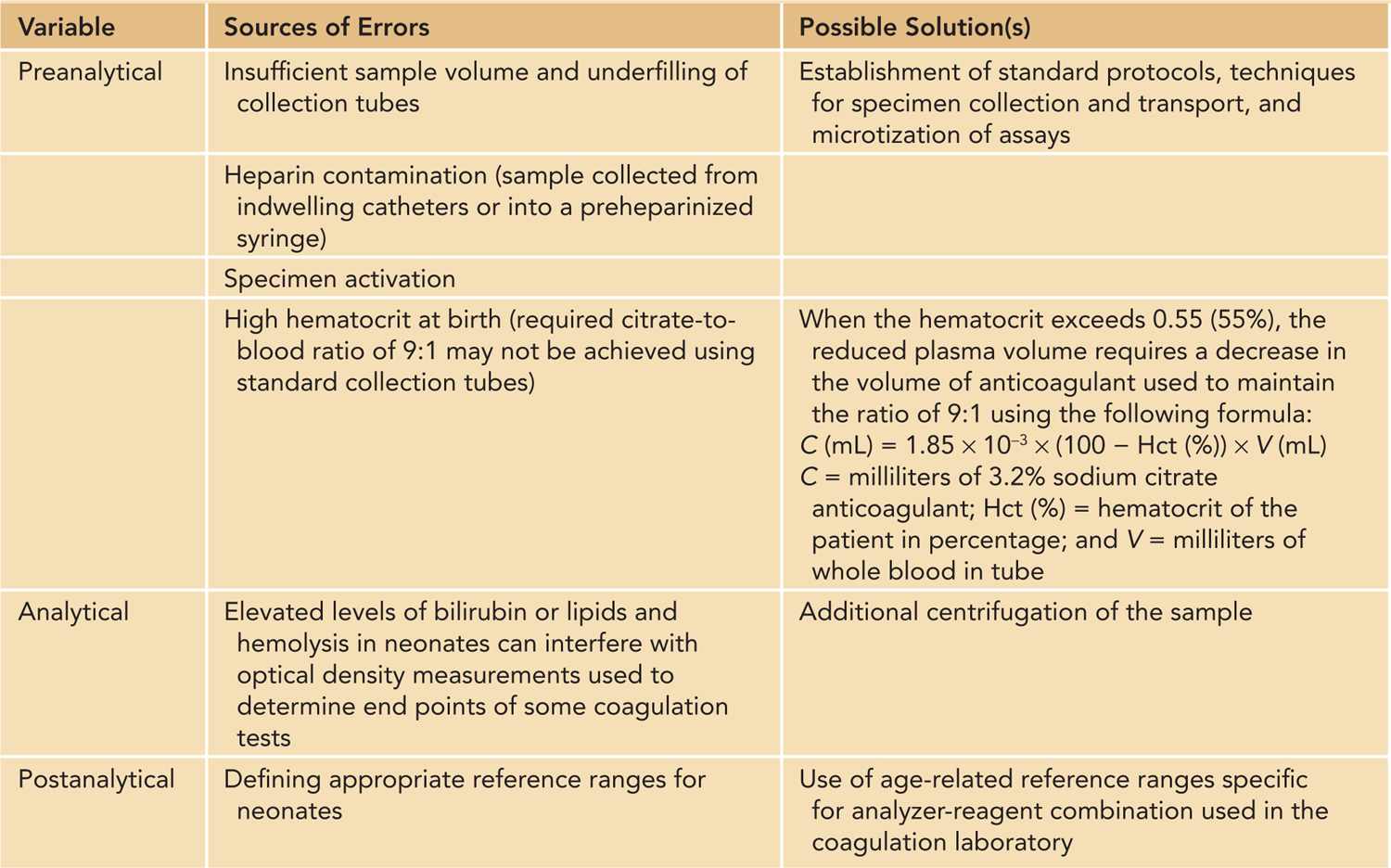
Bleeding disorders in neonates can be congenital or inherited but are more often acquired. It is clinically useful to classify bleeding disorders into those affecting primary hemostasis (blood vessels, vWF, and platelets) and those affecting secondary hemostasis (coagulation proteins). Disorders involving tertiary hemostasis (fibrinolytic system) are exceedingly rare. Acquired bleeding disorders can affect multiple components in the hemostatic system, which can give rise to complex hemostatic abnormalities. The evaluation of bleeding disorders in neonates can be challenging due to subtle or nonspecific clinical presentations and difficulties in both obtaining adequate specimens for coagulation testing and interpreting the results of these tests in the context of developmental hemostasis. A systematic approach and involvement of a pediatric hematologist are vital to establish the diagnosis of and therapeutic approach to a bleeding disorder. A complete history and physical examination is an essential first step that can direct further laboratory workup (Table 33-2). The laboratory evaluation of neonates with suspected bleeding disorders is best carried out in a rational stepwise fashion (Figure 33-2) in which an initial hemostatic screen is performed, which is followed by additional testing if an abnormality is identified on the initial screen. Additional testing is also indicated if the clinical suspicion remains high despite a normal hemostatic screen that cannot exclude several less-common or milder bleeding disorders (Figure 33-2). Moreover, several potential pitfalls should be kept in mind regarding laboratory evaluation of hemostasis in neonates6 (Table 33-3). While many of these issues can be overcome, the major challenge in hemostatic testing in neonates remains the establishment of appropriate neonatal reference values that are analyzer and reagent specific and at the same time clinically relevant (ie, reference ranges that can accurately identify “disease”).6
Table 33-2 Pertinent History and Physical Examination Findings in Neonatal Bleeding Disorder

Table 33-3 Special Considerations in Neonatal Hemostatic Laboratory Testing
NEONATAL PLATELET DISORDERS
This group of disorders results when platelets are deficient in number (thrombocytopenia), functionally defective (thrombocytopathy), or both. Typical manifestations include petechiae (singly or in crops), bruising, and hemorrhage from mucosal surfaces. Bleeding from a heel stick puncture is often the first clinical indicator of an issue. Although generally rare, serious bleeding can complicate some of the more severe platelet defects. In this section, we review platelet disorders that present in neonates.
Neonatal Thrombocytopenia
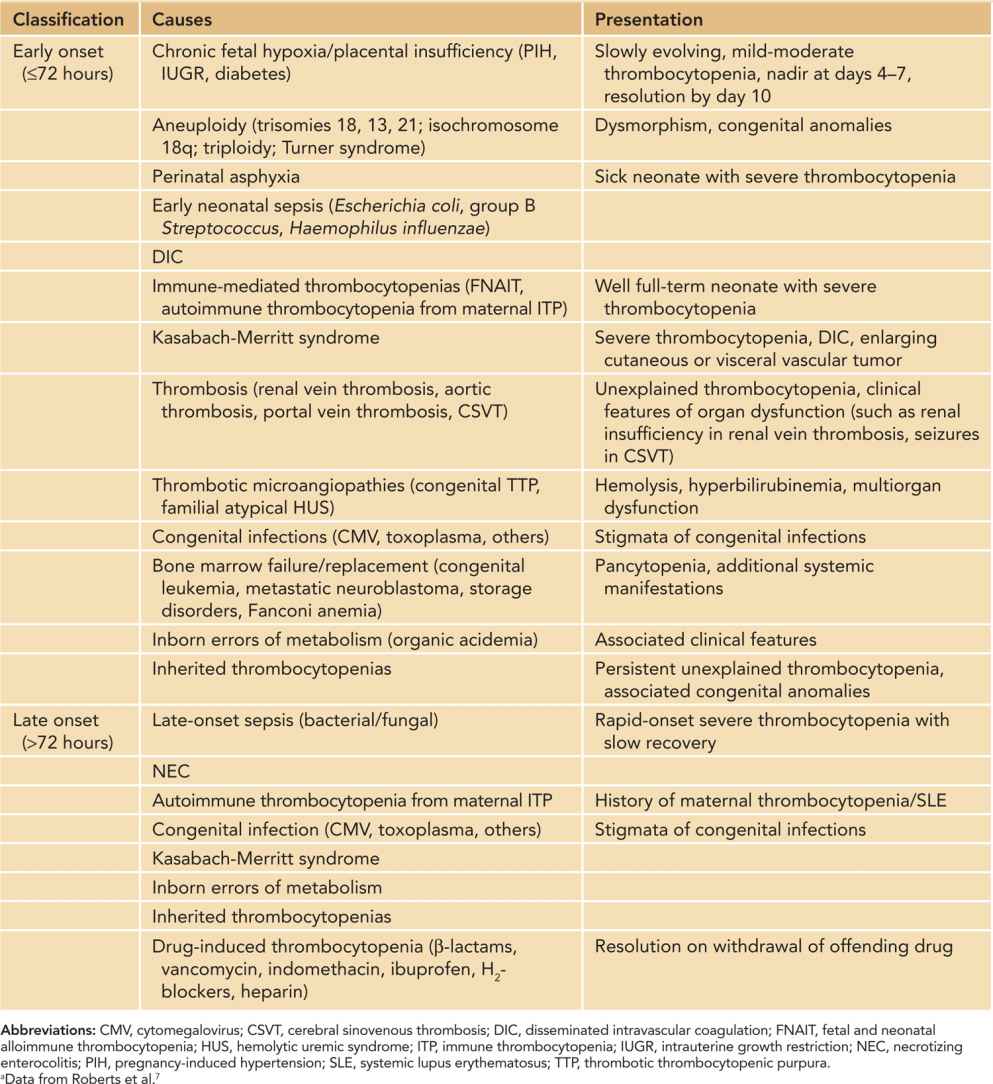
Thrombocytopenia in a neonate of any viable gestational age is traditionally defined7 as a platelet count of less than 150 × 109/L. However, recently gestational and postnatal age-specific reference intervals for platelet counts have been described that may need to be considered when defining thrombocytopenia in premature neonates.8 Thrombocytopenia is one of the most frequent hematological disorders encountered in the sick neonate.9 This is evidenced by a fairly high incidence among neonates admitted to the neonatal intensive care unit (NICU) (22%–35%) compared to a relatively low overall incidence (0.7%–0.9%).10 The incidence is inversely proportional to gestational age or birth weight.11 Severe thrombocytopenia (platelet count < 50 × 109/L) is less common, affecting 5%–10% of all neonates and 14% of extremely low birth weight (ELBW) infants.7,12 The predisposition of neonates to develop thrombocytopenia in response to illness is likely due to limited ability of the neonatal megakaryopoietic axis to increase platelet production in response to platelet consumption.13 While there are a large number of disease processes that have been associated with thrombocytopenia in neonates, impaired platelet production is the prevailing mechanism in most cases.9 Consumption or sequestration underlie thrombocytopenia in 25%–35% of cases.9 The differential diagnosis, and consequently diagnostic evaluation, of neonates with thrombocytopenia is usually based on onset (early onset ≤ 72 hours and late onset > 72 hours of life), overall presentation, and natural course (Table 33-4). While the most common identifiable causes are chronic fetal hypoxia in early-onset thrombocytopenia and sepsis and necrotizing enterocolitis (NEC) in late-onset thrombocytopenia, no etiology is identified in a significant proportion of thrombocytopenic ELBW neonates.11
Table 33-4 Causes of Neonatal Thrombocytopeniaa
Neonatal Thrombocytopenic Disorders Requiring Special Attention
Immune-Mediated Thrombocytopenias
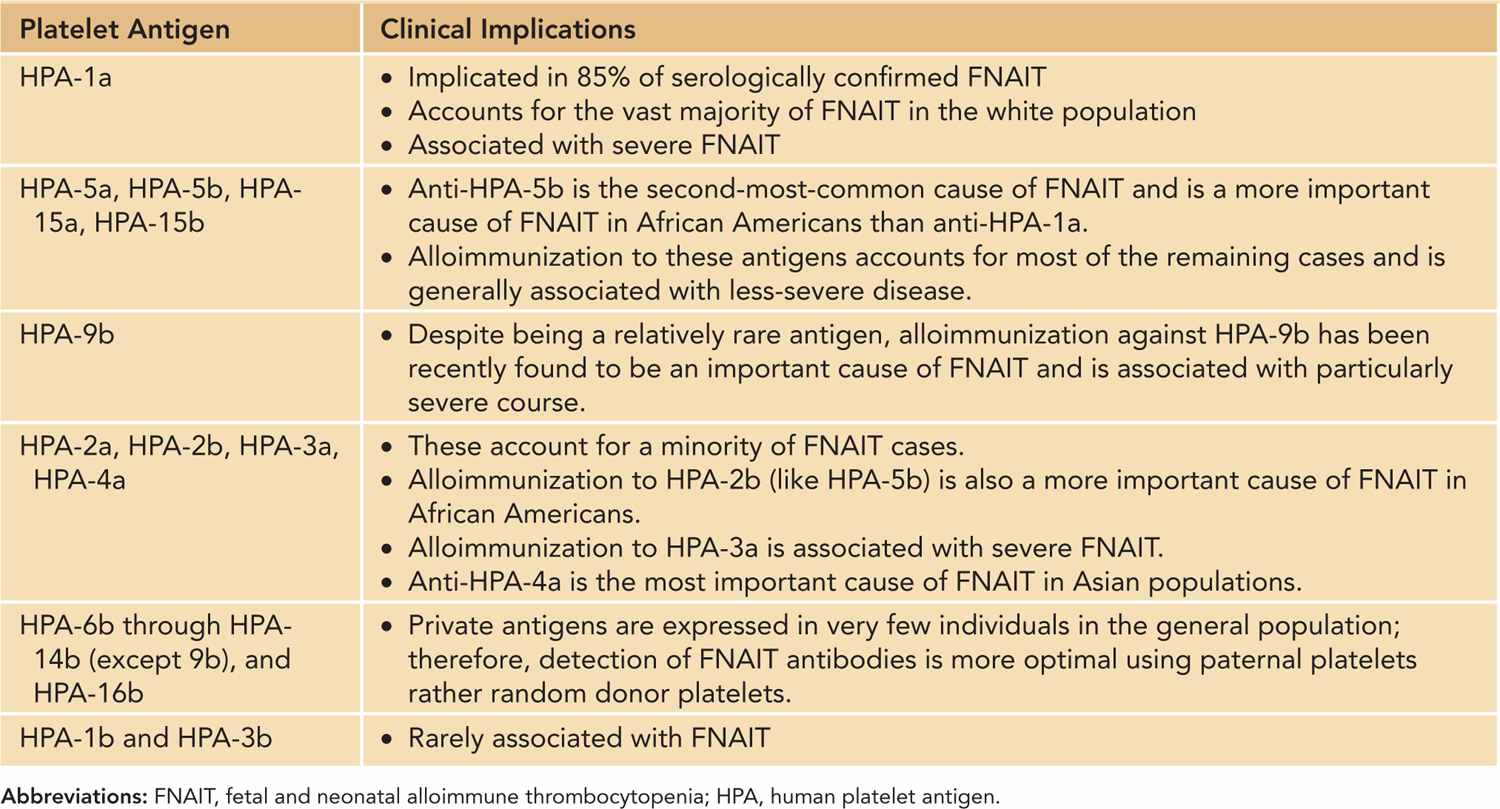
Fetal and Neonatal Alloimmune Thrombocytopenia (FNAIT): FNAIT is the most common cause of severe neonatal thrombocytopenia and of intracranial hemorrhage (ICH) in term neonates.14 FNAIT occurs with an estimated incidence of 1/1000–2000 live births.15 FNAIT results from transplacental passage of maternal antibodies to fetal platelets expressing paternal antigens that the mother lacks. Fetomaternal incompatibility involving certain human platelet antigens (HPAs) specifically expressed by platelets leads to the production of an immunoglobulin (Ig) G alloantibody, which then crosses the placenta, often in early pregnancy, binding to fetal platelets and resulting in fetal and neonatal thrombocytopenia. Genetic incompatibility to platelet antigens is necessary, but not sufficient, for FNAIT, as the clinical syndrome is seen for less than what would be expected based on genetic frequency alone.14 Thus, other factors, including genetic immune response modifiers and HLA alleles, must be important in the pathophysiology.16 The degree of significant bleeding in FNAIT is striking compared to other thrombocytopenic disorders, such as immune thrombocytopenia (ITP); alloantibody-mediated secondary effects may be responsible, including platelet dysfunction and megakaryocytic damage that causes prolonged thrombocytopenia and endothelial injury (eg, HPA-1a antigen is expressed by endothelial cells).16 HPA frequencies vary among different ethnic groups, and the severity of FNAIT can correlate with the specific HPA incompatibility identified (Table 33-5).
Table 33-5 Platelet Antigens and FNAIT
Fetal and neonatal alloimmune thrombocytopenia should be strongly considered in one of two clinical scenarios: In the first scenario, an otherwise-healthy neonate, born after an uneventful pregnancy and delivery, exhibits petechiae or widespread purpura associated with severe thrombocytopenia (platelet count < 50 × 109/L) within 24–48 hours of birth. For the second scenario, ICH, porencephaly, or ventriculomegaly is discovered during fetal life. About 10%–20% of clinically recognized cases will have ICH, which can be asymptomatic, discovered radiographically or symptomatically. Of ICH, 50%–75% occurs in utero and can be fatal (one-third of cases) or result in significant neurologic sequelae. To confirm a clinical diagnosis of FNAIT, two laboratory criteria need to be satisfied: platelet antigen incompatibility demonstrated by genotyping (polymerase chain reaction [PCR] techniques) and phenotyping (flow cytometry, various immunoassay methods) and evidence of maternal alloantibody directed against the identified alloantigen demonstrated by various immunoassays (eg, enzyme-linked immunosorbent assay [ELISA]) and using maternal serum tested against paternal platelets (preferably) or platelets from normal donors with known platelet alloantigen phenotypes.14 In addition, neuroimaging of all neonates with suspected FNAIT is recommended because some will have asymptomatic central nervous system bleeding, requiring more aggressive treatment measures.
Autoimmune Thrombocytopenia in Infants Born to Mothers With ITP: Neonatal ITP due to transplacental passage of maternal IgG antiplatelet autoantibodies occurs in up to 25% of mothers who develop primary or secondary ITP (due to systemic lupus erythematosus) during pregnancy.7,17 The risk of severe thrombocytopenia and ICH in affected neonates is significantly lower compared to FNAIT (<15% and <2%, respectively). Importantly, maternal platelet counts during pregnancy cannot reliably predict the risk of neonatal thrombocytopenia; however, a maternal platelet count less than 50 × 109/L at delivery and a history of severe thrombocytopenia in a previous neonate may be useful indicators of the likelihood of significant neonatal thrombocytopenia complicating current pregnancy.18,19 Typically, the nadir platelet count in affected neonates is observed between days 2 and 5 after birth, with resolution by day 7; however, some neonates may continue to have thrombocytopenia secondary to maternal ITP for months, necessitating long-term monitoring. Evaluation of affected neonates should include serial platelet counts and screening transcranial ultrasonography (US; if platelet count is < 50 × 109).
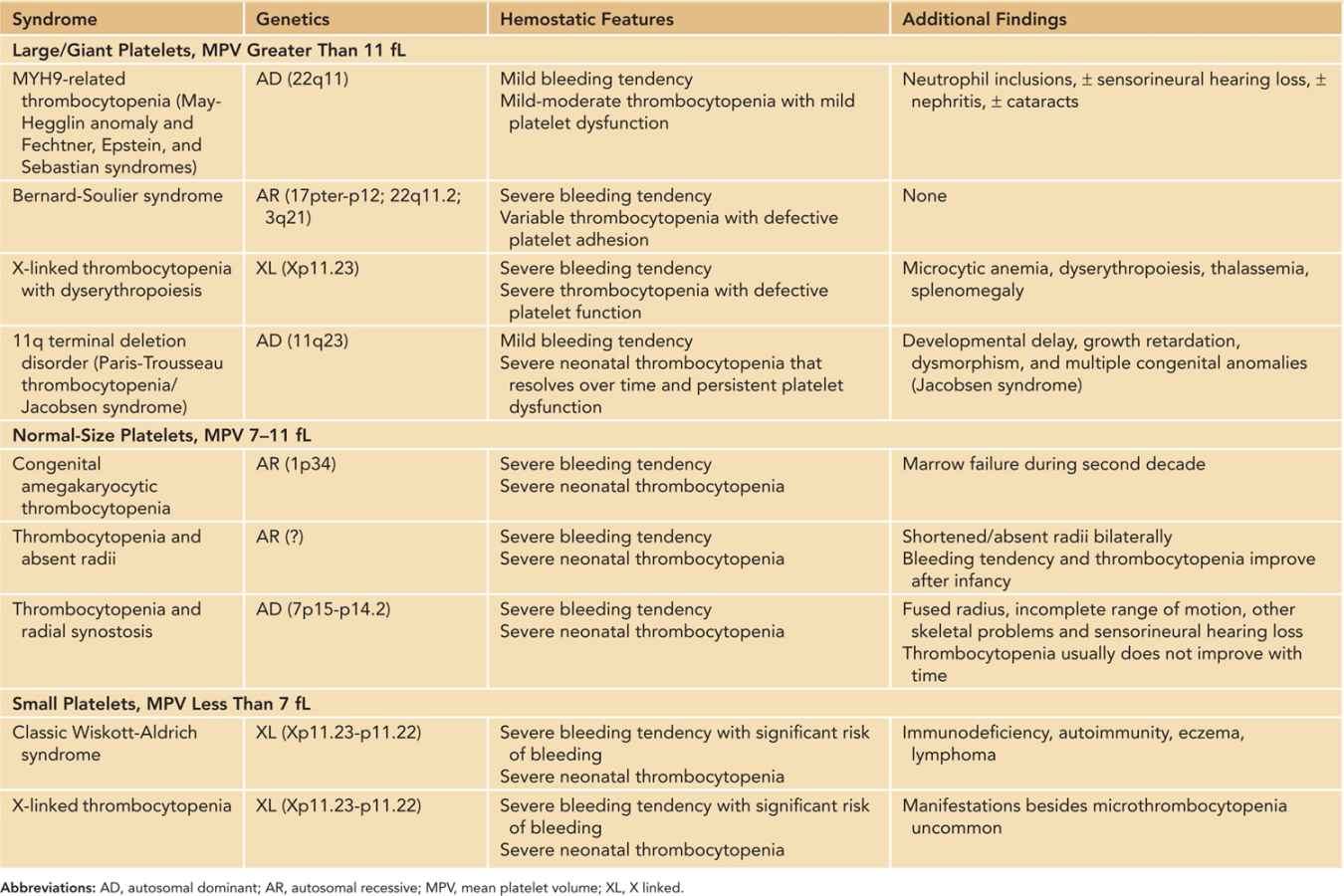
Inherited Thrombocytopenias: The inherited thrombocytopenias (Table 33-6) belong to a rare group of heterogeneous disorders with highly variable bleeding tendency. Although far less common than acquired forms, inherited thrombocytopenias should be considered in neonates with (1) persistent unexplained thrombocytopenia since birth; (2) bleeding tendency disproportionate to the platelet counts; (3) family history of thrombocytopenia; (4) typical congenital anomalies/dysmorphism; and (5) suggestive blood smear abnormalities (consistently large or small platelets, abnormal platelet granules, or neutrophil inclusion “Döhle bodies”). Except for Wiskott-Aldrich syndrome (WAS) and X-linked thrombocytopenia (XLT), in which increased consumption of platelets in addition to defective production are implicated, the thrombocytopenia in these disorders is generally thought to be the result of impaired production. In addition, associated platelet dysfunction can be identified in many inherited thrombocytopenias, which are often responsible for the significant bleeding tendency that is out of proportion to the measured platelet count—a characteristic feature of some of these disorders (eg, Bernard-Soulier syndrome [BSS]). The diagnostic evaluation of these disorders can be challenging and should be conducted by a clinician with expertise in this area and directed by clinical manifestations, physical examination findings, and results of complex laboratory tests, some of which are only available in research laboratories. Genetic or molecular studies are needed to confirm the diagnosis in some inherited platelet disorders (such as WAS/XLT).
Table 33-6 Inherited Thrombocytopenic Syndromes That Can Present in the Neonatal Period
Platelet Function Disorders
Platelet function disorders include a large number of disorders with either inherited or acquired mechanisms (Table 33-7). These disorders pose diagnostic challenges because the tests needed to fully evaluate platelet function can be complex, requiring a great deal of expertise, and are often restricted to specialized coagulation or research laboratories (Figure 33-3). As a group, these disorders present as primary hemostatic defects of variable severity characterized by mucocutaneous bleeding (bruising, petechiae). Although most congenital platelet function defects rarely cause pathologic bleeding in the neonatal period, the severe platelet function disorders like Glanzmann thrombasthenia (GT) and Bernard-Soulier syndrome (BSS) can present in neonates (Table 33-8). Some of these congenital disorders are associated with thrombocytopenia, while others cause isolated platelet dysfunction with normal platelet counts. For more information about these disorders, excellent reviews are available.20,21
Table 33-7 Causes of Platelet Function Defects