Benign Vulvovaginal Disorders
Lori A. Boardman
Colleen M. Kennedy
Vulvovaginal symptoms are common, often chronic, and can significantly interfere with women’s sexual function and sense of well-being. Obtaining expert medical advice to deal with such complaints can be frustrating. The goal of this chapter is to provide a working knowledge of appropriate diagnostic and counseling tools and a structured framework in which to approach the evaluation and management of common, but frequently problematic, as well as uncommon vulvovaginal disorders. To that end, commonly encountered infectious conditions, such as various manifestations of human papillomavirus (HPV)-associated disease, herpetic infections, and the acute vaginitides, as well as chronic forms of common infections, such as vulvovaginal candidiasis and bacterial vaginosis, will be discussed. Other topics to be covered include autoimmune disorders, including lichen sclerosus and lichen planus; inflammatory disorders, including contact dermatitis and lichen simplex chronicus; an overview of common benign cysts and pigmented vulvar lesions; and lastly, vulvodynia.
Vulvovaginal Infections (Viral, Fungal, Bacterial, Other)
Human Papillomavirus and External Genital Warts
Genital HPV, the most common sexually transmitted viral infection, is associated with a number of vulvar epithelial disorders such as genital warts, vulvar intraepithelial neoplasia (VIN), and some vulvar carcinomas. In excess of 100 HPV subtypes have been identified, of which more than 30 are specific to the anogenital tract. Although low-risk HPV types are implicated in the development of genital warts (90% of which are associated with types 6 and 11) and oncogenic HPV 16 is commonly found in warty-basaloid or undifferentiated VIN, the routine use of HPV testing in the diagnosis of vulvar HPV-related disease is not currently recommended.
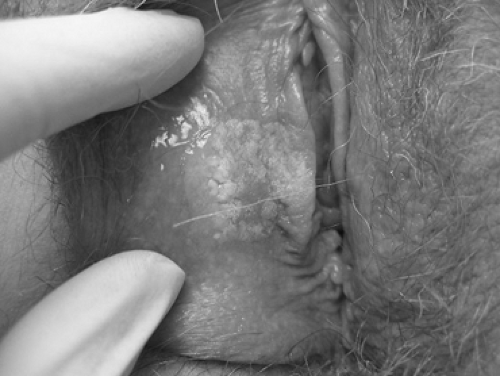
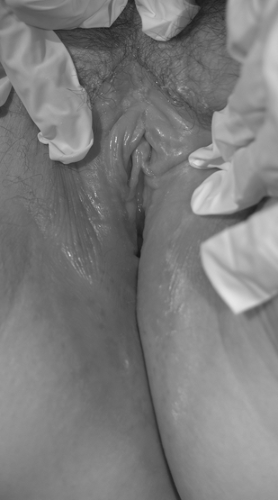
Genital warts often present as bumps or growths on the moist surfaces of the vulvovaginal or perianal mucosa and can cause itching, burning, pain, or bleeding. Distinguishing warts from vulvar neoplasia based on appearance alone is not always possible. In general, hyperpigmented, indurated, fixed, or ulcerative lesions and lesions that do not respond to treatment or worsen during treatment should be biopsied, as VIN can present as red, white, dark, raised, or eroded lesions (Figs. 35.1, 35.2). If the diagnosis is uncertain or the patient is immunocompromised, biopsy should be undertaken. Women with genital warts should undergo routine cervical cancer screening. The use of HPV testing, a change in the frequency of cervical cytologic screening, or cervical colposcopy are not indicated in the presence of genital warts. If exophytic cervical warts are detected during examination or if vulvar neoplasia is confirmed by biopsy, referral for colposcopic evaluation is indicated.
Although spontaneous regression of genital warts can occur in up to 30% of affected patients, many patients opt for therapy. Currently recommended treatment options for genital warts focus largely on physical destruction or removal of visible disease. These include podofilox 0.5% solution or gel (patient-applied) as well as the provider-administered therapies: cryotherapy, podophyllin resin 10% to 25%, trichloroacetic or bichloroacetic acid 80% to 90%, surgical removal, and laser therapy (Table 35.1). Such therapies have been the mainstay of treatment, along with imiquimod, a targeted antiviral therapy. Local irritation (e.g., pain, burning, and soreness), erythema,
edema, and, at times, ulceration can result from the use of any of these medications. Surgical excision or laser vaporization should be reserved for patients with extensive disease.
edema, and, at times, ulceration can result from the use of any of these medications. Surgical excision or laser vaporization should be reserved for patients with extensive disease.
Podofilox (keratolytic) and imiquimod (antiviral) are newer therapeutic choices that patients and providers find both efficacious and preferable. Clearance rates for the two treatments are similar and compare favorably to any of the treatments listed previously. The added benefit of imiquimod, as demonstrated in numerous studies, has been its significantly lower recurrence rates (9% to 19%).
Intralesional interferon, a medication with antiproliferative, antiviral, and immunomodulatory properties, has, unlike imiquimod, demonstrated limited efficacy and is not recommended for first-line therapy in treating either warts or VIN. Its use, however, can be considered an alternative regimen for the treatment of external genital warts. Finally, with regard to pregnancy, the data regarding long-term sequelae in infants and children infected with HPV at delivery are inconsistent and do not justify routine cesarean section in women with genital warts.
Intralesional interferon, a medication with antiproliferative, antiviral, and immunomodulatory properties, has, unlike imiquimod, demonstrated limited efficacy and is not recommended for first-line therapy in treating either warts or VIN. Its use, however, can be considered an alternative regimen for the treatment of external genital warts. Finally, with regard to pregnancy, the data regarding long-term sequelae in infants and children infected with HPV at delivery are inconsistent and do not justify routine cesarean section in women with genital warts.
TABLE 35.1 Treatment of Genital Warts | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||
Herpes Simplex Virus
Herpes simplex virus (HSV) is the most common cause of ulcerative genital lesions. Less common causes of ulcerative vulvar disease are shown in Table 35.2. HSV-1 typically causes orolabial lesions and herpes keratitis, while HSV-2 primarily infects the anogenital tract via genital–genital sexual contact with an infected partner who is shedding virus. Infection with HSV-2 is common, involving 25% of adults aged 30 and older in the United States, although most remain unaware that they are infected. Significant crossover of subtypes, however, does exist, with estimates indicating HSV-1 infection in approximately 20% of current cases of genital herpes in the United States.
Primary infection with HSV presents with multiple, superficial, painful ulcerations of the lower genital tract and tender inguinal adenopathy. Systemic symptoms (including fever, malaise, and headache) occur in approximately 60% of women. Periodic reactivation results in either clinically apparent lesions or, more commonly, asymptomatic infection. Recurrent lesions, in general, are less severe. Approximately half of patients who recognize recurrences report prodromal symptoms in the hours to days before the onset of genital lesions. In recurrences, the duration of viral shedding is shorter, and few lesions are present. Asymptomatic viral shedding, however, accounts for most HSV transmission.
While 90% of patients with a first documented episode of genital HSV-2 infection will have a least one recurrence within the first year following diagnosis, 38% will have six or more recurrences, and 20% will suffer ten or more recurrences. Compared with HSV-2 infections, genital HSV-1 infections recur less frequently. With time, recurrences do decrease, irrespective of viral type and use or nonuse of suppressive therapy. The major complications of HSV infection include multiple neurologic sequelae, the most common of which is aseptic meningitis, seen in approximately one third of women with primary infection. Immunocompromised patients are at risk for prolonged or severe episodes of genital, perianal, or oral herpes. HSV-related lesions are common in HIV infection, and shedding is increased in this population, even in the presence of antiretroviral therapy.
Clinical history and physical findings often guide the diagnosis of genital herpes. Viral culture can be used to confirm the diagnosis, realizing its inherent limitations. While roughly 95% of the early vesicular lesions of herpes will grow HSV, only 70% of ulcerative lesions and 30% of crusted lesions will yield evidence of the virus. Type-specific serologic testing for HSV may be of use in confirming infection in patients with crusted lesions or with an unclear history of past infection for whom the diagnosis would be clinically helpful (e.g., women with HIV infection or those with partners known to be HSV positive). Finally, any woman presenting with genital ulcers should undergo HIV testing as part of her evaluation.
The cornerstone of treatment for HSV infection consists of the nucleoside analogue antiviral medications: acyclovir, famciclovir, and valacyclovir. All may be used in the treatment of primary and nonprimary first episodes, episodic recurrent episodes, and as suppressive therapy in patients with six or more annual outbreaks. Suppressive therapy also can be considered in patients with less frequent recurrences, as the use of such therapy has been shown to be safe, efficacious, and associated with improvement in quality-of-life indicators. All three medications significantly decrease the number of days of viral shedding, local pain and dysuria, hasten the healing of lesions, and decrease the production of new lesions 48 hours after initiating therapy. No one therapy emerges superior, although valacyclovir can be administered less frequently due to enhanced absorption following oral administration. Topical therapy with acyclovir cream or ointment has not been shown to be effective. Current recommendations for antiviral regimens are included in Table 35.3.
Tinea Cruris (Dermatophytosis)
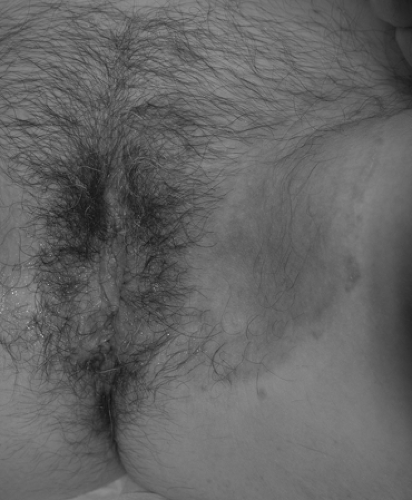
A superficial fungal infection caused most commonly by Epidermophyton floccosum and Trichophyton rubrum or less commonly by Trichophyton mentagrophytes, tinea cruris presents as a pruritic, pale red skin eruption with annular scaling margins. Over the course of weeks, the rash will spread slowly from the groin. The infection usually is found in young adults and results from either sexual transmission or autoinoculation. The rash associated with tinea cruris typically is well defined and sharply marginated, spreading in an annular pattern peripherally from the groin. In women, the rash normally first appears in the hair-bearing portion of the vulva but can extend to the inguinal area and buttocks (Fig. 35.3). The diagnosis can be confirmed by microscopic evidence of hyphae. A sample can be obtained by scraping scale from the leading edge of the rash onto a glass slide. A drop of 10% potassium hydroxide is placed on the slide, followed by a cover slip.
While more than 25 randomized controlled trials document the efficacy of antifungal therapy, few evaluate the two different classes of antifungals used for tinea cruris head to head. Based on available evidence, however, no superior treatment emerges between the topical allylamines or allylamine derivatives (terbinafine available as a 1%
emulsion gel, cream, or spray/solution; naftifine 1% cream, or butenafine 1% cream) as compared with the azole antifungals (such as 1% clotrimazole cream or 2% miconazole cream). While the allylamines are significantly more costly, they do allow for a shorter duration of treatment (once daily for 1 week compared with 2 to 3 weeks for the topical azoles).
emulsion gel, cream, or spray/solution; naftifine 1% cream, or butenafine 1% cream) as compared with the azole antifungals (such as 1% clotrimazole cream or 2% miconazole cream). While the allylamines are significantly more costly, they do allow for a shorter duration of treatment (once daily for 1 week compared with 2 to 3 weeks for the topical azoles).
TABLE 35.2 Less Common Causes of Genital Ulcers in the United States | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 35.3 Antiviral Management Regimens for Herpes Simplex Viral Infections | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||
Vulvovaginal Candidiasis
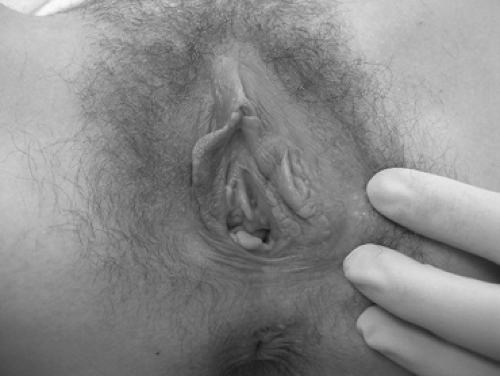
Estimates indicate that 75% of sexually active women will experience the symptoms of itching, irritation, soreness, dyspareunia, and dysuria associated with candidiasis; 45% of women will experience two or more vaginal yeast infections; and 5% will suffer from recurrent (four or more episodes a year) vulvovaginal candidiasis. Most candidal infections are sporadic and, in over 90% of cases, are caused by Candida albicans. Uncomplicated vulvovaginal candidiasis is defined as sporadically or infrequently occurring, of mild to moderate intensity, most likely secondary to C. albicans, and involving immunocompetent women. Complicated candidiasis includes women with recurrent infection, severe manifestations of candidiasis (extensive vulvar erythema, edema, excoriation, and fissure formation) (Fig. 35.4), or infection with nonalbicans species as well as women with uncontrolled diabetes, debilitation or immunosuppression, or those who are pregnant. Women with complicated candidiasis, particularly recurrent disease, account for the majority of physician visits by women for chronic vaginitis, and it is this group that will be the focus of the remaining discussion. Women with recurrent candidiasis present a challenge both in terms of evaluation and treatment. Although nonalbicans species should be suspected and culture clearly is warranted in this population, it should be remembered that repeated vulvovaginitis most commonly occurs with persistent C. albicans. Nonalbicans Candida sp. occur in approximately 10% to 20% of women with recurrent disease. While testing for diabetes or immunosuppression is often suggested for women with recurrent disease, in only a minority of patients will such testing be profitable. For example, postmenopausal women with recurrent yeast infections should be assessed for the presence of diabetes. Recurrent vulvovaginal candidiasis, however, should not be used as the sole indication to prompt HIV testing.
A reliable diagnosis cannot be made based on symptoms or signs in the absence of corroborative laboratory data. Determination of the vaginal pH as well as direct microscopy should be performed. A vaginal pH <4.5 is consistent with candidiasis, although an elevated pH does not exclude this diagnosis, as concomitant infection with bacterial vaginosis (BV) or trichomoniasis, for example, can
raise the vaginal pH. The sensitivity of KOH microscopy, while reportedly better than that of saline microscopy, has been estimated to range from 38% to 83%; as a result, the absence of yeast argues against but cannot exclude candidiasis. Given the limitations of current office testing and the increased likelihood of nonalbicans species among women with recurrent disease, initial evaluation should include vaginal cultures obtained on at least two occasions, preferably while the patient is symptomatic. If two consecutively obtained cultures are negative for Candida sp., the diagnosis of recurrent disease can be excluded.
raise the vaginal pH. The sensitivity of KOH microscopy, while reportedly better than that of saline microscopy, has been estimated to range from 38% to 83%; as a result, the absence of yeast argues against but cannot exclude candidiasis. Given the limitations of current office testing and the increased likelihood of nonalbicans species among women with recurrent disease, initial evaluation should include vaginal cultures obtained on at least two occasions, preferably while the patient is symptomatic. If two consecutively obtained cultures are negative for Candida sp., the diagnosis of recurrent disease can be excluded.
For uncomplicated candidiasis, a variety of topical and oral medications can be used (Table 35.4). Treatment results in relief of symptoms and mycologic cure rates in 80% to 90% of patients who complete therapy. While cure rates are similar between topical and oral therapies, patients strongly prefer the oral route of administration. Side effects of topical medications are limited largely to local reactions (burning, itching, and irritation), while oral fluconazole is associated more commonly with nausea and headache.
Conventional antifungal therapy is not as effective in patients with complicated candidiasis. In general, patients with severe disease and patients who are pregnant, immunosuppressed, or debilitated will require longer courses of topical therapy (7 to 14 days) or if not pregnant, sequential doses of fluconazole (taken on days 1 and 4). For recurrent infection with C. albicans, the patient should be treated with the same regimen used for severe disease followed by maintenance therapy with 6 months of either oral medication (weekly fluconazole in a 100-mg, 150-mg, or 200-mg dose) or clotrimazole (200 mg topically twice a week or a 500-mg vaginal suppository weekly). In a recent randomized controlled trial of suppressive therapy with fluconazole, women with recurrent disease on maintenance medication for 6 months, compared with those on placebo, were significantly more likely to remain diseasefree at both 6 months (91% vs. 36%) and at follow-up off medication 6 months later (43% vs. 22%). Lastly, while often a concern, complicated candidiasis caused by fluconazole-resistant C. albicans remains uncommon.
Nonalbicans infections (e.g., C. glabrata, C. krusei, C. parapsilosis, and C. tropicalis) do not typically respond to treatment with fluconazole, often secondary to an intrinsic resistance to this medication. Recommended first-line therapy, then, is a 7- to 14-day course of a nonfluconazole azole (oral or topical). If treatment fails, a 2-week course of nightly boric acid vaginal suppositories (600 mg in a 0 gel capsule) is recommended. Both clinical and mycologic cure rates with this regimen approach 70%. If not successful, however, the patient should be referred to a specialist
for further management. For recurrent disease, suggested maintenance therapy regimens include a 6-month course of a nightly nystatin vaginal suppository or twice-weekly boric acid vaginal suppositories.
for further management. For recurrent disease, suggested maintenance therapy regimens include a 6-month course of a nightly nystatin vaginal suppository or twice-weekly boric acid vaginal suppositories.
TABLE 35.4 Treatment of Uncomplicated Vulvovaginal Candidiasis, Bacterial Vaginosis, and Trichomoniasis | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Bacterial Vaginosis
BV is a complex polymicrobial disorder characterized by decreased lactobacilli, primarily the hydrogen peroxide–producing strains Lactobacillus crispatus and L. jensenii and increased colonization by facultative or strictly anaerobic microorganisms such as Gardnerella vaginalis, Peptostreptococcus sp., Prevotella sp., Mobiluncus sp., Bacteroides sp., and Mycoplasma hominis. Although BV is the most common cause of vaginitis, its prevalence varies. For example, black, non-Hispanic women as well as women reporting a history of douching in the past 6 months were found to be at increased risk of BV in a recent study using data from the National Health and Nutrition Examination surveys. In other studies, rates vary from 5% to 26% among pregnant women worldwide, while higher rates have been documented in women attending sexually transmitted disease (STD) clinics (24% to 37%). From an epidemiologic standpoint, BV is thought to be sexually associated at minimum, although multiple studies have failed to show a benefit from partner treatment in preventing recurrent BV.
BV has been associated with a number of reproductive complications, including preterm labor, premature rupture of membranes, and low birth weight. Possible gynecologic sequelae consist of a variety of postsurgical complications, including postabortal endometritis and vaginal cuff cellulitis or abscess formation following hysterectomy as well as pelvic inflammatory disease (PID). Finally, BV appears to be a risk factor for the acquisition and transmission of HIV infection as well as the acquisition of HSV-2 infection.
The gold standard for diagnosing BV is the Nugent score, derived based on the Gram stain and used primarily in the research setting. For the practitioner, the diagnosis of BV most often is made clinically by using vaginal pH and the wet or saline preparation. To secure the diagnosis, three of four Amsel criteria must be met: (a) homogeneous, thin, white discharge that smoothly coats the vaginal walls;
(b) vaginal pH >4.5; (c) a fishy odor of vaginal discharge before or after the addition of 10% potassium hydroxide (positive amine or “whiff” test); and (d) the presence of more than 20% of epithelial cells as clue cells on microscopy. Compared with the Nugent score, Amsel criteria have a sensitivity of 92% and a specificity of 77%, although evidence suggests that the combination of any two of these clinical criteria will achieve similar sensitivity and specificity. Women found to have evidence consistent with the diagnosis of BV on a Pap smear should be evaluated clinically only in the presence of symptoms and treated if Amsel criteria are fulfilled.
(b) vaginal pH >4.5; (c) a fishy odor of vaginal discharge before or after the addition of 10% potassium hydroxide (positive amine or “whiff” test); and (d) the presence of more than 20% of epithelial cells as clue cells on microscopy. Compared with the Nugent score, Amsel criteria have a sensitivity of 92% and a specificity of 77%, although evidence suggests that the combination of any two of these clinical criteria will achieve similar sensitivity and specificity. Women found to have evidence consistent with the diagnosis of BV on a Pap smear should be evaluated clinically only in the presence of symptoms and treated if Amsel criteria are fulfilled.
The Centers for Disease Control and Prevention (CDC) currently recommend three treatment protocols for BV—oral metronidazole, topical metronidazole, or topical clindamycin (Table 35.4). Women using metronidazole should avoid alcohol intake during the course of therapy and for 24 hours thereafter due to the possibility of a disulfiramlike reaction. Clindamycin cream can weaken latex condoms and diaphragms, an effect that can persist up to 5 days following treatment. In addition, therapy with either metronidazole or clindamycin is associated with an elevated risk of candidiasis (10% to 30%).
Although there is no published data on the effect of treatment for BV prior to hysterectomy, a randomized, double-blind study of treatment prior to first-trimester abortion showed a significant reduction in PID among those in the treatment arm. Based on such evidence, the CDC recommends screening and treatment for BV before invasive gynecologic procedures. Although not explicitly recommended, the CDC STD treatment guidelines for PID state that many experts recommend oral metronidazole in addition to the standard outpatient regimen for PID.
Short-term studies indicate that while initial cure rates for BV range from 70% to 90%, recurrence rates are high. Within 3 months of therapy, recurrences are seen in approximately 15% to 30% of women, although rates of 60% to 70% have been documented. Despite promising evidence, there currently is no clearly superior evidence-based method of treatment for recurrent BV. Multiple regimens have been suggested, including lactobacilli replacement therapy, maintenance acetic acid gel, and prolonged therapy with metronidazole. In a placebo-controlled trial of L. crispatus vaginal capsules, recolonization with L. crispatus occurred in the majority of women with recurrent BV, and in those colonized, relapses were significantly less likely to occur compared with those not colonized. Among women who failed to become recolonized, recurrences were more common among those on placebo. Currently, Phase III clinical trials are under way to assess the efficacy of recolonization with vaginal-specific lactobacilli (i.e., L. crispatus and L. jensenii).
In 2006, Sobel published data on a multicenter trial of metronidazole gel among 157 women with BV. All participants initially received a 10-day course of nightly 0.75% metronidazole gel and if cured were then randomized to either gel or placebo twice weekly for 16 weeks. An intent-to-treat analysis done at week 16 showed that 26% of patients using the gel had a recurrence of BV compared with 59% of those on placebo.
Trichomoniasis
Trichomonas vaginalis, a sexually transmitted parasite, is the most common sexually transmitted infection in the United States aside from HPV. Although often considered of minor importance compared with other sexually transmitted infections, growing evidence of the adverse health consequences of trichomoniasis suggests otherwise. Upper genital tract infections (including those following delivery, abortion, or surgical procedures), PID, tubal infertility, preterm delivery, and increased risk of HIV transmission have all been described in association with trichomoniasis. Risk factors for infection include a change in sexual partners, frequent intercourse, three partners or more in the past month, and another coexistent STD.
Symptomatic women may present with abnormal discharge, itching, burning, or postcoital spotting. An elevated vaginal pH is typical, and motile trichomonads and signs of inflammation often are present on microscopy. The sensitivity of the wet mount, however, ranges from 45% to 60%, and culture (either in Diamond’s medium or via use of a two-chambered plastic bag culture system [InPouch, BioMed Diagnostics, White City, OR]) remains the standard for diagnosis.
Treatment recommendations are included in Table 35.4. Patients taking metronidazole should be advised to refrain from alcohol consumption for 24 hours after completion of therapy; for tinidazole, abstinence should be maintained for 72 hours. Sexual partners should be treated as well, and intercourse should be avoided until both partners are cured (i.e., therapy completed and both partners asymptomatic). Cure rates for metronidazole and tinidazole are high (90% to 95% for metronidazole, 86% to 100% for tinidazole), although randomized trials comparing the 2-g doses of both medications point to increased parasitologic cure and resolution of symptoms with tinidazole. It should be noted that metronidazole gel is not recommended for treatment of trichomoniasis secondary to poor efficacy (<50%).
Metronidazole-resistant trichomoniasis has been reported, although the resistance seen is usually of low level. If treatment fails with the 2-g metronidazole dose (and reinfection is not present), the patient should be treated with either the 7-day oral regimen of metronidazole or the 2-g tinidazole in a single dose. If failure occurs with either one of these doses, clinicians should consider treatment with the 2-g dose of either metronidazole or tinidazole daily for 5 days. If persistence occurs, consultation with an infectious disease specialist is warranted (this service as well as T. vaginalis




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree