Fig. 21.1
Surgeon console
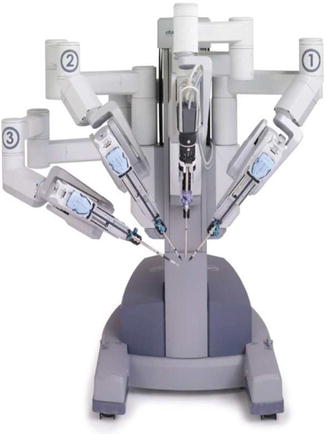
Fig. 21.2
Patient cart
Robotic Technology
It enables the surgeon to be more precise, improve their technique, and enhance their capability in performing complex minimally invasive surgery.
1.
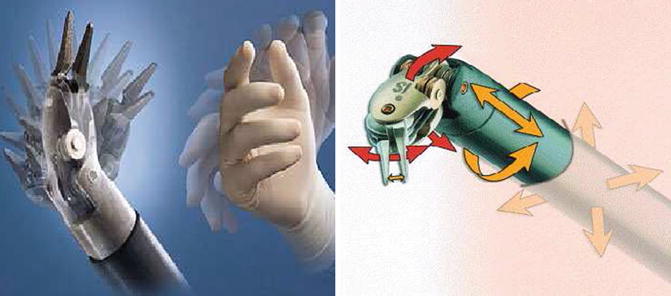
Binocular stereoscopic 3D vision (Fig. 21.3) with stability of camera and 10× magnification.
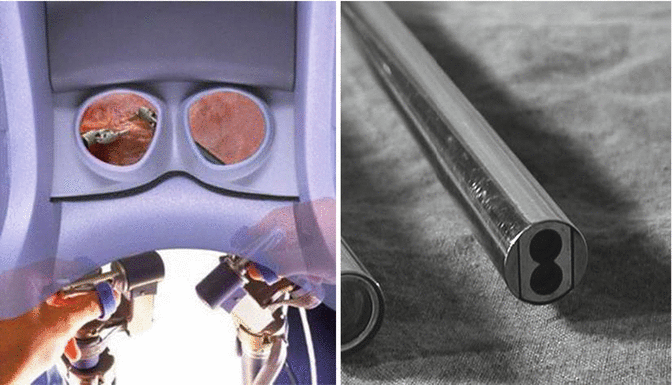

Fig. 21.3
Binocular stereoscopic vision and camera
The robotic system also allows the surgeon to better visualize anatomy, which is especially critical when working around delicate and confined structures like in the pelvis, chest, or abdomen. This allows surgeons to perform radical cancer surgeries with superior oncological outcome.
2.
It mimics the human hand in its flexible movement and also overcomes its limitations, like elimination of hand tremors. Despite the widespread use of laparoscopic surgery, adoption of laparoscopic techniques, for the most part, has been limited to a few routine procedures. This is due mostly to the limited capabilities of traditional laparoscopic technology, including standard video and rigid instruments. Surgeons have been slow to adopt to laparoscopy for complex procedures because they generally find fine-tissue manipulation such as dissecting and suturing to be more difficult (Table 21.1). Intuitive technology, however, enables the use of robot for complex procedures (Table 21.2). The robot allows for seven degrees of motion vs. the limited 4° of motion in laparoscopy. Robotic technology eliminates the fulcrum effect of laparoscopy (the robotic arms imitate the movements of the surgeon’s hand).
Table 21.1
Disadvantages of laparoscopy
Steep learning curve |
Limited dexterity |
Counterintuitive motion |
Two-dimensional field |
Limited depth perception |
Ergonomic difficulty |
Table 21.2
Advantages of robotic technology
Binocular stereoscopic 3D vision |
Stable high-definition camera with 10× magnification |
EndoWrist instrumentation – increased dexterity |
Extremely easy and fast suturing and knotting intracorporeally |
Surgeons sit and operate at ease with arms rested |
Multitasking instrumentations |
Option of harmonic scalpel |
Three arms in addition to the camera arm |
Filters human tremor |
Ergonomics with equal access with both left- and right-sided ports |
3.
Motion scaling and precision surgical movements improve the quality of surgery.
4.
Extremely easy and allows fast suturing and knotting.
5.
Multitasking instrumentation decreases operative time.
6.
Surgeon sits and operates at ease which decreases fatigue, translating to safe surgery.
Surgical Technique
Preoperative Preparation
Patient takes clear liquids a day prior to surgery. On the night before the surgery, proctoclysis enema and two Dulcolax (bisacodyl) tablets are given per oral. We do not administer polyethylene glycol with electrolytes (Peglec) for bowel preparation as it causes dilatation of bowel.
Port Placement and Instrumentation
Port placement and instrumentation are shown in Figs. 21.5, 21.6, and 21.7, respectively. VCARE (Vaginal–Cervical Ahluwalia Retractor–Elevator) uterine manipulator is fixed to the cervix after placing the patient in lithotomy position. Intraoperatively, it helps in manipulating the uterus. A 12 mm camera port is placed 3 cm above the umbilicus in the midline with optical trocar. The rest of the ports are placed after insufflating the abdomen with gas and marking the port measurements. Arm one (8 mm) port is placed on the patient’s right side, 3–5 cm below and at least 8 cm lateral to the camera port. Arm two (8 mm) port is placed on the patient’s left side, 8 cm lateral and 3–5 cm below the level of the camera port. The third arm (8 mm) port is placed on the patient’s right side, 2 cm above anterior superior iliac spine and 8 cm away from the first port. The assistant port (12 mm) is placed on the patient’s left side, slightly cephalad to the camera port on an arc at the midpoint between the camera port and the instrument arm two port.
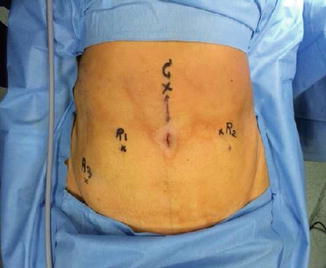
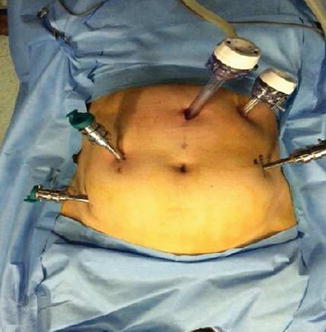
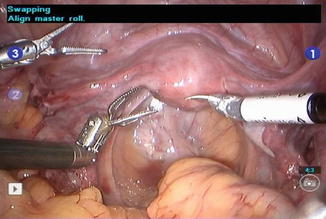

Fig. 21.5
Abdominal marking of port placement

Fig. 21.6
Port placement

Fig. 21.7
Robotic instruments showing dexterity
Zero degree scope is used for all the steps, except for para-aortic lymph node dissection where 30° down scope is used. In arm one, hot shears (monopolar curved scissors) are used; in arm two, fenestrated bipolar forceps; and in arm three, prograsp forceps.
Patient positioning is shown in Fig. 21.8, and docking of the patient cart is shown in Figs. 21.9 and 21.10.
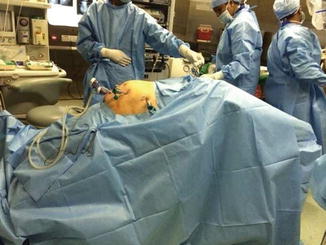
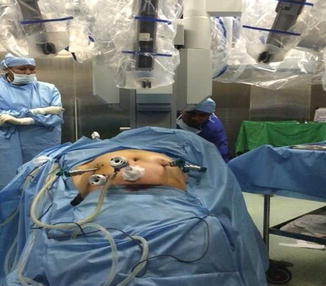

Fig. 21.8
Patient positioning: head end lowered to 45°

Fig. 21.9
Robot (patient cart) is docked

Fig. 21.10
Widely spaced arms after docking between legs
After placing all the ports, the patient is positioned before docking the robot. Head end is lowered completely, and all the bowel loops are taken toward the upper abdomen. Pelvic wash is given, and fluid is taken for cytological examination.
Surgical Steps
Dissection is done in a circular fashion from one round ligament to the other.
Step 1: The uterus is retracted to the patient’s left side with the help of the uterine manipulator. Dissection starts with incising the peritoneum over infundibulopelvic triangle, isolating the ureter and ovarian pedicle. Then, the round ligament is transected near the inguinal ring with hot shear (monopolar diathermy). Incision is extended anteriorly into the anterior leaf of the broad ligament up to the lateral uterovesical junction. Coagulate and transect the right uterine pedicle and cardinal ligament. Careful attention to the course of the ureter at all times must be kept in mind.
Step 2: The urinary bladder is lifted up with the third arm, and the uterus is retroverted with the help of the uterine manipulator and second arm. The vesicouterine groove is identified, and the bladder is dissected away from the uterus, and adhesions if any are dissected with the cold knife (hot shear).
Step 3: Left side isolation of the ureter and dissection of the round ligament are done similar to Step 1. Both side ovarian pedicles are coagulated with bipolar diathermy but not divided until complete dissection is done.
Step 4: Posterior part dissection is done by separating the rectum from the uterus with the division of uterosacral ligaments on either side. The course of the ureter must be noted during this step.
Step 5: Anterior and posterior colpotomies are done by incising over the colpotomy ring. Finally, both the ovarian pedicles are divided. Specimen is delivered through the vagina by pulling out the uterine manipulator, and abdominal pneumatic pressure is maintained by packing the vagina with an adequate-sized mop inside a surgical hand glove.
Step 6: Bilateral pelvic lymphadenectomy (Figs. 21.11, 21.12, and 21.13) is done by exposing pararectal and paravesical spaces. A separate specimen bag is used for lymph nodes of either side, and specimen is delivered through the vagina. Para-aortic lymph node dissection is done when indicated. Vaginal cuff is closed with a 15 cm long self-retaining polydioxanone (monofilament, violet) barbed suture, and uterosacral ligaments are included laterally.
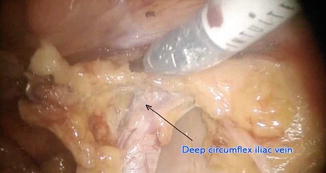
Fig. 21.11
Pelvic lymphadenectomy: distal boundary
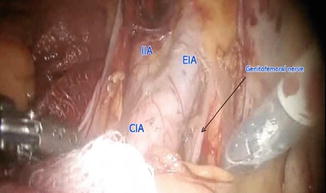
Fig. 21.12
Pelvic lymphadenectomy: lateral and proximal boundary
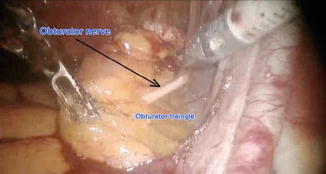
Fig. 21.13
Pelvic lymphadenectomy: inferior boundary
The role of systematic pelvic lymphadenectomy is an issue of current debate. Excision of suspicious or enlarged nodes is important to exclude metastasis. A more selective and tailored lymphadenectomy approach is now recommended to avoid systematic overtreatment [6]. No randomized trial data supports full lymphadenectomy [7] although some retrospective studies have suggested that it is beneficial [8]. A subset of patients may not benefit from lymphadenectomy, but it is difficult to preoperatively identify these patients because of the uncontrollable variable of change in grade and depth of invasion in final histopathology.
As the grade of the tumor increases, accuracy of intraoperative evaluation of myometrial invasion by gross examination decreases. Therefore, frozen section examination for evaluation of the histology, size of primary, grade, and depth of invasion is important. Pending further trials, pelvic lymphadenectomy is done in all patients. Para-aortic lymphadenectomy is indicated in high-risk patients. High-risk patients are with tumor size >2 cm, deep myometrial invasion, positive pelvic nodes, Grade 3 tumor, and high-risk (clear cell, papillary serous, squamous, or undifferentiated) histology.
Anatomical spaces in pelvic dissection:
1.
Paravesical space
2.
Pararectal space
Pelvic lymphadenectomy: anatomical boundaries
Distally – deep circumflex iliac vein
Proximally – common iliac vessels
Laterally – genitofemoral nerve
Inferiorly – obturator fossa
Para-aortic lymphadenectomy
Boundaries
Superiorly – renal vein
Inferiorly – common iliac vessels
Laterally – ureter
Efficacy of Laparoscopy
The Gynecologic Oncology Group (GOG) has completed a phase III randomized study (lamina-associated polypeptide (LAP) 2) comparing laparoscopy vs. laparotomy in endometrial cancer [9]. Patients with clinical stage I to IIA uterine cancer were randomly assigned to laparoscopy (n = 1,696) or open laparotomy (n = 920), including hysterectomy, salpingo-oophorectomy, pelvic cytology, and pelvic and para-aortic lymphadenectomy. Laparoscopy was initiated in 1,682 patients and completed without conversion in 1,248 patients (74.2 %). Conversion from laparoscopy to laparotomy was secondary to poor visibility in 14.6 %, metastatic cancer in 4.1 %, bleeding in 2.9 %, and other causes in 4.2 %. Laparoscopy had fewer moderate to severe postoperative adverse events than laparotomy (14 % vs. 21 %, respectively; P = .0001) but similar rates of intraoperative complications, despite having a significantly longer operative time (median, 204 vs. 130 min, respectively; P = .001). Hospitalization of more than 2 days was significantly lower in laparoscopy vs. laparotomy patients (52 % vs. 94 %, respectively; P = .0001). They concluded that laparoscopic surgical staging for uterine cancer is feasible and safe in terms of short-term outcomes and results in fewer complications and shorter hospital stay. Time to recurrence was the primary end point, with non-inferiority defined as a difference in recurrence rate of less than 5.3 % between the two groups at 3 years. The recurrence rate at 3 years was 10.24 % for patients in the laparotomy arm, compared with 11.39 % for patients in the laparoscopy arm, with an estimated difference between groups of 1.14 % (90 % lower bound, −1.278; 95 % upper bound, 3.996) [10]. Although this difference was lower than the prespecified limit, the statistical requirements for non-inferiority were not met because of a lower-than-expected number of recurrences in both groups. The estimated 5-year overall survival was almost identical in both arms at 89.8 %. These results, combined with previous findings from this study of improved QOL and decreased complications associated with laparoscopy, are reassuring to patients and allow surgeons to reasonably suggest this method as a means to surgically treat and stage patients with presumed early-stage endometrial cancers.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree