Bag-Valve-Mask Ventilation
Christopher King
Stacy L. Reynolds
Introduction
The first steps in most pediatric resuscitations are to establish a patent airway and provide adequate ventilatory support. Although definitive management of a respiratory emergency often involves endotracheal intubation, this may not be possible initially if the appropriate equipment is unavailable or properly trained personnel are not present. Even in settings where intubation can be performed rapidly, the patient may require respiratory support before the procedure. It is in such situations that bag-valve-mask (BVM) ventilation is indicated. Patients requiring assisted or controlled ventilation may be managed on an immediate basis with minimal preparation using this procedure. In most cases, BVM ventilation serves as a temporizing measure to be used before intubation and during intervals between intubation attempts. It is sometimes possible, however, to support a patient through a brief apneic episode (e.g., oversedation) with BVM ventilation alone. Furthermore, the utility of this procedure as a primary means of respiratory support for children in the prehospital setting was demonstrated in a seminal study by Gausche et al. (1). In a large, randomized trial comparing BVM ventilation alone with endotracheal intubation, these authors found no differences in survival or neurologic outcome among pediatric patients requiring transport to the hospital.
BVM ventilation is a procedure that many health care providers—including physicians, nurses, respiratory therapists, and paramedics—are called on to perform. It is undoubtedly the procedure most commonly used by medical personnel in the initial management of pediatric respiratory emergencies. But this should not be taken to mean that BVM ventilation is a simple procedure. Most practitioners who care for critically ill children can relate experiences during which performing manual ventilation proved far more difficult than endotracheal intubation. In addition, the ability of inexperienced operators to effectively perform BVM ventilation with pediatric patients may be limited (2). For these reasons, as with any airway intervention, the attention of the team leader in a pediatric resuscitation should never be far from the proficiency with which BVM ventilation is being performed.
Anatomy and Physiology
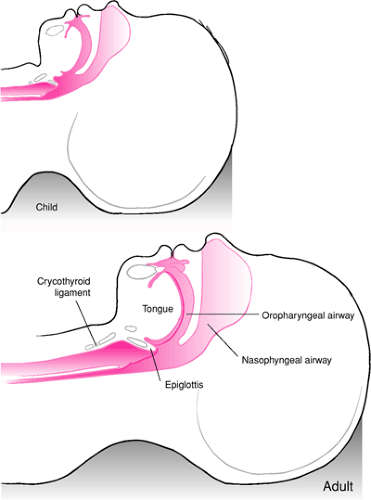
A number of anatomic differences exist between pediatric and adult patients that have important clinical implications regarding the use of BVM ventilation (Fig. 14.1). Not surprisingly, these differences are most apparent among infants and become less pronounced as the patient matures through older childhood and adolescence. One such characteristic is that the tongue of an infant or younger child is larger relative to the oral cavity than in the case of an adult. In addition, the tongue has a more rostral (superior) location in relation to the roof of the mouth. The larger size and “higher” position of the tongue greatly increase the likelihood of airway obstruction when the patient is lying supine. An artificial airway may therefore be necessary to maintain a conduit for gas flow when performing BVM ventilation with a pediatric patient (see Chapter 13). Infants also exhibit obligate nasal breathing until about 3 to 5 months of age as a result of obstruction of the oropharyngeal airway as the tongue rests against the roof of the mouth. Occlusion of the nares because of stenosis or choanal atresia, as well as transient causes such as blood, vomitus, or secretions, can produce profound respiratory distress and even asphyxia in an infant (3,4). In such cases, BVM ventilation is an effective means of bypassing the obstruction and providing gas exchange via the oropharynx.
The immature laryngeal cartilages of the pediatric airway are more compliant, allowing wider variation in the airway diameter in response to compressive or distending forces (5,6).
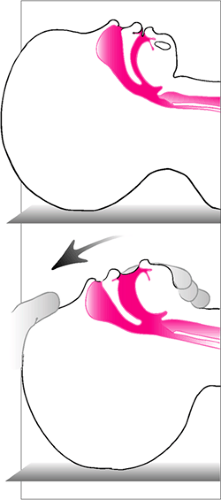
As a result, pediatric patients with partial airway obstruction are more prone to develop dynamic collapse of the extrathoracic airway as a result of high negative intraluminal pressures, increasing the work of breathing. Manual ventilation is often effective in such situations, because this negative pressure is reduced or eliminated (see below). Infants and children also have greater laxity of the cervical spine and neck musculature (7,8,9). This can lead to excessive rotation of the head during attempts to establish a patent airway, which can result in complete airway obstruction (Fig. 14.2).
As a result, pediatric patients with partial airway obstruction are more prone to develop dynamic collapse of the extrathoracic airway as a result of high negative intraluminal pressures, increasing the work of breathing. Manual ventilation is often effective in such situations, because this negative pressure is reduced or eliminated (see below). Infants and children also have greater laxity of the cervical spine and neck musculature (7,8,9). This can lead to excessive rotation of the head during attempts to establish a patent airway, which can result in complete airway obstruction (Fig. 14.2).
As described in Chapter 16, the optimal position of the head and neck for airway patency is the so-called sniffing position. The neck is slightly flexed while the head is rotated into extension. This is said to be the position assumed when one is “sniffing the air,” and it results in the most favorable alignment of the oral, pharyngeal, and tracheal axes (10) (Fig. 14.3). With adolescents and adults, it is generally necessary to place a small towel roll or pad under the head to achieve the proper degree of neck flexion. However, because infants have a relatively large occiput, such measures are not usually required (Fig. 14.4). In fact, it may be helpful to place a pad or towel roll under the shoulders to elevate the torso slightly if an infant has an especially prominent occiput.
The relative immaturity of the pediatric diaphragm, intercostal muscles, and ribs can also impact on the use of BVM ventilation. The chest wall of an infant or child is significantly
more compliant than that of an adolescent or adult (11). As a result, there is less “protective” resistance provided by the chest wall, which results in a higher incidence of barotrauma during BVM ventilation. In addition, pediatric patients have a greater tendency to develop early respiratory muscle fatigue, potentially leading to respiratory arrest. This is primarily the result of the immature diaphragm having a lower content of Type I muscle fibers (slow twitch, high oxidative capacity), which confer fatigue resistance (12). The infant’s diaphragm is also more horizontal and therefore less efficient in expanding the lungs.
more compliant than that of an adolescent or adult (11). As a result, there is less “protective” resistance provided by the chest wall, which results in a higher incidence of barotrauma during BVM ventilation. In addition, pediatric patients have a greater tendency to develop early respiratory muscle fatigue, potentially leading to respiratory arrest. This is primarily the result of the immature diaphragm having a lower content of Type I muscle fibers (slow twitch, high oxidative capacity), which confer fatigue resistance (12). The infant’s diaphragm is also more horizontal and therefore less efficient in expanding the lungs.
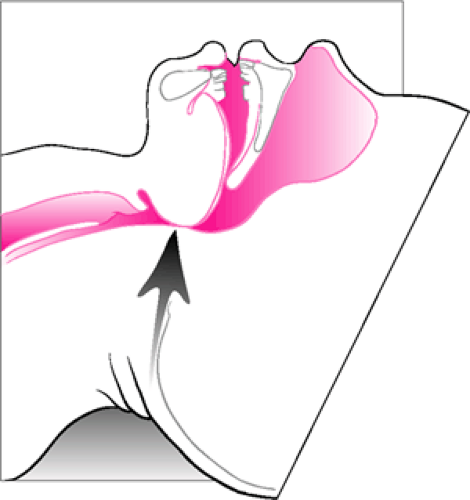 Figure 14.2 Excessive extension of the neck in an attempt to establish airway patency can actually cause or worsen obstruction. |
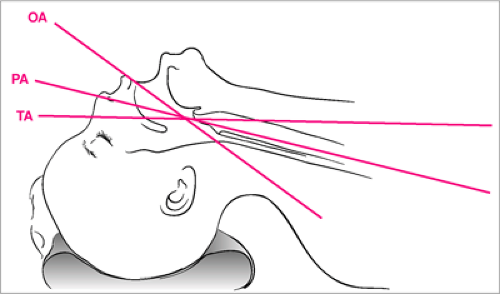 Figure 14.3 The sniffing position results in the most favorable alignment of the oral, pharyngeal, and tracheal axes for airway patency. A small pad or towel roll placed under the head may be needed to achieve the necessary flexion of the neck for an older child or adolescent (see also Fig. 16.3). |
Certain congenital abnormalities of the face and airway can make BVM ventilation difficult or impossible to perform. For example, achieving an adequate mask seal may not be possible if the child has micrognathia or other midfacial anomalies. This is also true for patients with severe trauma to the face or mandible. In addition, administering manual ventilation may be problematic for children with macroglossia due to difficulty maintaining a patent airway. In such cases, providing positive pressure ventilation to treat respiratory failure may require immediate endotracheal intubation.
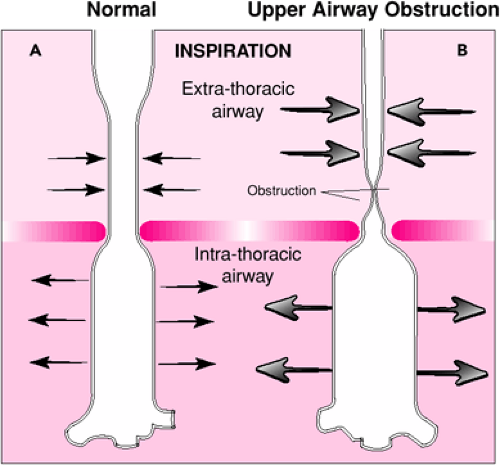
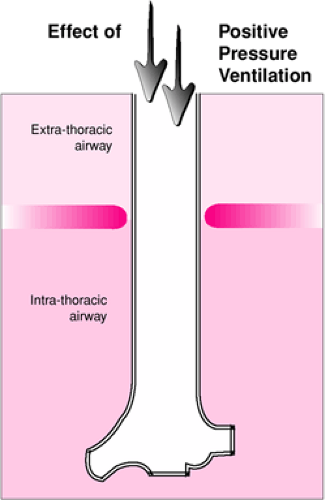
Use of BVM ventilation may be affected by several characteristics of pediatric respiratory physiology. As with endotracheal intubation, BVM ventilation is a useful means of providing respiratory support because it offers a method of delivering positive pressure ventilation. Although normal spontaneous ventilation and positive pressure ventilation both result in oxygen delivery to the body, in many ways these two processes have opposing physiologic effects. Spontaneous respiration occurs when the negative intrathoracic pressure generated by contraction of the respiratory muscles (primarily the diaphragm) causes expansion of the lungs and passive movement of air into the upper airway. As the term implies, positive pressure ventilation with a BVM circuit involves forced air entry through the upper airway and into the lungs, which is produced by an externally applied pressure. One clinically important result of this difference is the contrasting effects these two modes of ventilation have on the extrathoracic airway (Fig. 14.5). With spontaneous respiration, the extrathoracic airway experiences negative intraluminal pressures (relative to atmospheric pressure) during inspiration. This negative pressure may be significantly increased with partial airway obstruction and can result in dynamic collapse of the airway proximal to the obstruction, which can worsen the patient’s respiratory distress and increase the work of breathing (5). Controlled ventilation with a BVM circuit tends to “stent” the extrathoracic airway open, even when partial obstruction is present, as the airway experiences positive intraluminal pressures throughout inspiration (Fig. 14.6).
Physiologic differences between spontaneous ventilation and positive pressure ventilation may also play a role in some adverse consequences associated with BVM ventilation. For example, the elevated intrathoracic pressure that occurs with positive pressure ventilation reduces venous return to the heart and can cause a decrease in cardiac output (13), although interestingly this may be less pronounced in children than adults (14).
This effect normally has clinical significance only for patients with hypovolemia or borderline cardiac function. Consequently, such patients who receive BVM ventilation may require increased intravenous fluid administration to maintain stable hemodynamics. In addition, BVM ventilation can exacerbate ventilation-perfusion (VQ) mismatching (15,16). With spontaneous ventilation, the lung bases receive the majority of both air entry and blood flow during inspiration; ventilation and perfusion are therefore well matched. When a patient receives BVM ventilation, the distribution of air entry is determined primarily by compliance of the lung parenchyma, which is greatest in the apices. As a result, while blood continues to flow preferentially to the bases, the majority of air entry now goes to the apices, resulting in VQ mismatch. This accounts for the spontaneously breathing patient who may have a decrease in oxygen saturation when assisted ventilation with a BVM circuit is initiated. For this reason, the delivered oxygen concentration should be maximized whenever manual ventilation is performed.
This effect normally has clinical significance only for patients with hypovolemia or borderline cardiac function. Consequently, such patients who receive BVM ventilation may require increased intravenous fluid administration to maintain stable hemodynamics. In addition, BVM ventilation can exacerbate ventilation-perfusion (VQ) mismatching (15,16). With spontaneous ventilation, the lung bases receive the majority of both air entry and blood flow during inspiration; ventilation and perfusion are therefore well matched. When a patient receives BVM ventilation, the distribution of air entry is determined primarily by compliance of the lung parenchyma, which is greatest in the apices. As a result, while blood continues to flow preferentially to the bases, the majority of air entry now goes to the apices, resulting in VQ mismatch. This accounts for the spontaneously breathing patient who may have a decrease in oxygen saturation when assisted ventilation with a BVM circuit is initiated. For this reason, the delivered oxygen concentration should be maximized whenever manual ventilation is performed.
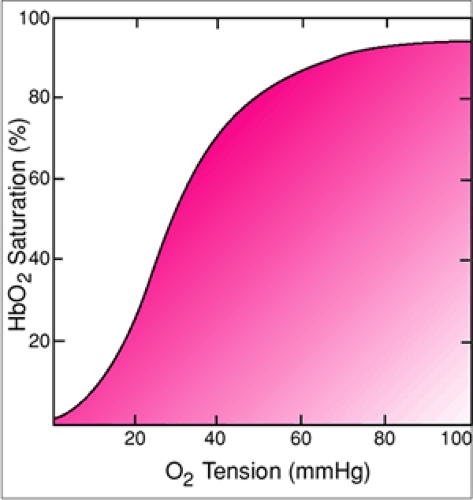
One of the more unwelcome events during BVM ventilation of a pediatric patient is the sudden, dramatic decline in oxygen saturation that results when oxygen delivery is inadequate. This is also related to a unique characteristic of pediatric physiology; the rate of oxygen consumption among infants and children is significantly higher than among adults. In fact, the baseline oxygen consumption of a neonate is approximately twice that of an adult (17). As a result, if the concentration of oxygen delivered by manual ventilation is insufficient for any reason (e.g., unrecognized mask leak, rebreathing of expired gases), the pO2 of a pediatric patient will drop more rapidly than it will with an adult (18). The rapidity of the decline appears to be even more pronounced among children with upper respiratory infections (19). Because the oxygen dissociation curve has a flat segment at higher oxygen saturations, the reading of the pulse oximeter remains relatively stable initially despite the fact that the pO2 is falling (Fig. 14.7). If this problem is not recognized, oxygen saturation will suddenly decline precipitously when the pO2 reaches the steep zone of the curve. This diminished margin for error makes the timely recognition of problems leading to inadequate oxygen delivery an important priority with pediatric patients.
Indications
BVM ventilation is used to provide temporary ventilatory support until definitive management can be undertaken or the patient resumes spontaneous respiration. Indications for the use of BVM ventilation can be divided into two general
categories: problems of the airway and problems with ventilation (see also Chapter 16). Airway indications for BVM ventilation include the various causes of upper airway obstruction affecting pediatric patients that can lead to significant respiratory compromise. For example, partial airway obstruction resulting from bacterial and viral infections such as croup, bacterial tracheitis, and retropharyngeal abscess may serve as an indication for BVM ventilation. As mentioned previously, infants and younger children may present with frank respiratory arrest resulting solely from obstruction caused by laxity of the pharyngeal musculature and posterior displacement of the tongue. Such patients normally have other medical problems (e.g., sepsis, toxic ingestion) that are causing a profoundly depressed mental status. In this situation, the use of BVM ventilation, combined with proper positioning and insertion of an artificial airway, offers an effective initial approach to managing the respiratory component of the overall presentation. Other obstructive processes that can be addressed using BVM ventilation include hematoma, supraglottic edema, and upper airway foreign body. For the patient with a rapidly expanding hematoma of the upper airway, BVM ventilation can be used in the early stage of management, but tracheal intubation should be performed as soon as the patient is stabilized. In addition, foreign bodies should normally be removed from the airway whenever possible before initiating BVM ventilation, because smaller objects may be propelled distally by the force of ventilations (see Chapter 51).
categories: problems of the airway and problems with ventilation (see also Chapter 16). Airway indications for BVM ventilation include the various causes of upper airway obstruction affecting pediatric patients that can lead to significant respiratory compromise. For example, partial airway obstruction resulting from bacterial and viral infections such as croup, bacterial tracheitis, and retropharyngeal abscess may serve as an indication for BVM ventilation. As mentioned previously, infants and younger children may present with frank respiratory arrest resulting solely from obstruction caused by laxity of the pharyngeal musculature and posterior displacement of the tongue. Such patients normally have other medical problems (e.g., sepsis, toxic ingestion) that are causing a profoundly depressed mental status. In this situation, the use of BVM ventilation, combined with proper positioning and insertion of an artificial airway, offers an effective initial approach to managing the respiratory component of the overall presentation. Other obstructive processes that can be addressed using BVM ventilation include hematoma, supraglottic edema, and upper airway foreign body. For the patient with a rapidly expanding hematoma of the upper airway, BVM ventilation can be used in the early stage of management, but tracheal intubation should be performed as soon as the patient is stabilized. In addition, foreign bodies should normally be removed from the airway whenever possible before initiating BVM ventilation, because smaller objects may be propelled distally by the force of ventilations (see Chapter 51).
As discussed previously, pediatric patients are prone to fatigue and may therefore develop respiratory failure or arrest from airway obstruction long before BVM ventilation would be difficult to administer. In other words, the obstruction is rarely “complete.” Thus the operator should not be dissuaded from making an initial attempt at manually ventilating an arrested or severely compromised patient suspected of having airway obstruction from one of the above mentioned processes. It is important to emphasize, however, that assisted ventilation with a BVM circuit is not indicated for a child with high-grade airway obstruction who is, at least momentarily, stable and breathing adequately. Such a patient should not be disturbed but should instead be managed expectantly with airway equipment close at hand until surgical and anesthesia personnel are involved. Otherwise, the agitation and distress caused by attempts to perform BVM ventilation—or other measures such as forced administration of face mask oxygen, venipuncture, etc., which may not be immediately necessary—can hasten respiratory failure or arrest as the struggling child rapidly fatigues.
The ventilatory indications for performing BVM ventilation include (a) nonobstructive causes of hypoventilation and respiratory arrest, (b) processes that lead to ineffective ventilation, and (c) insults to the lungs that cause impaired gas exchange. Hypoventilation and respiratory arrest in the pediatric population can result from a wide variety of neurologic, infectious, toxicologic, and metabolic disorders. In the ED, overwhelming sepsis and traumatic brain injury are two of the most common causes. Infants are especially prone to develop apnea as the first significant manifestation of an underlying illness. BVM ventilation should be initiated during a neonatal resuscitation whenever the heart rate is below 100 or the patient has apneic spells lasting 30 seconds or longer (see Chapter 36). Transient hypoventilation resulting from iatrogenic drug overdose (e.g., benzodiazepines for status epilepticus) can sometimes be managed with BVM ventilation alone until the patient resumes spontaneous respiration. Ineffective ventilation among pediatric patients is most frequently associated with processes that affect patency of the small airways, such as asthma and bronchiolitis. Fatigue leading to hypoxia and hypercarbia usually accounts for the child’s worsening condition. With these patients, BVM ventilation can be used to temporarily reduce the work of breathing, although in such situations, endotracheal intubation will usually prove necessary. The decision to initiate manual ventilation is based primarily on the clinician’s assessment of the severity of the patient’s respiratory compromise. Impaired alveolar gas exchange results from diverse etiologies, such as lung infection, near-drowning, and pulmonary contusion. With more severe and diffuse involvement of the lungs, the patient may manifest clinical findings consistent with respiratory distress syndrome. These children may develop profound hypoxemia and frequently require endotracheal intubation. When BVM ventilation is used in the initial management of such patients, the circuit should be configured to reliably deliver a concentration of oxygen near 100% and, if necessary, to administer positive end expiratory pressure (PEEP).
There are few contraindications to BVM ventilation. Relative contraindications include any problems that significantly undermine the effectiveness of the procedure, such as
congenital or traumatic facial deformities so severe that a mask seal is impossible to maintain or a high-grade airway obstruction that prevents delivery of adequate ventilatory support. If such a process becomes apparent when BVM ventilation is attempted, an alternate procedure (e.g., immediate endotracheal intubation, percutaneous transtracheal ventilation, or a surgical airway) should be performed. In addition, BVM ventilation should not be used for any significant length of time with a patient who is suspected of having a tension pneumothorax before the appropriate interventions for this process are undertaken. Continued delivery of positive pressure ventilation can further increase the elevated intrathoracic pressure. BVM ventilation can be used for immediate stabilization if such a patient is apneic, but needle thoracostomy and/or chest tube insertion (Chapter 29) should be performed as soon as possible.
congenital or traumatic facial deformities so severe that a mask seal is impossible to maintain or a high-grade airway obstruction that prevents delivery of adequate ventilatory support. If such a process becomes apparent when BVM ventilation is attempted, an alternate procedure (e.g., immediate endotracheal intubation, percutaneous transtracheal ventilation, or a surgical airway) should be performed. In addition, BVM ventilation should not be used for any significant length of time with a patient who is suspected of having a tension pneumothorax before the appropriate interventions for this process are undertaken. Continued delivery of positive pressure ventilation can further increase the elevated intrathoracic pressure. BVM ventilation can be used for immediate stabilization if such a patient is apneic, but needle thoracostomy and/or chest tube insertion (Chapter 29) should be performed as soon as possible.
The only situations that represent absolute contraindications to BVM ventilation are (a) the initial management of a newborn suspected of having significant meconium aspiration during delivery who does not appear vigorous (20) and (b) the presence of a congenital diaphragmatic hernia in a newborn with respiratory distress, both of which require immediate endotracheal intubation. For the newborn with significant meconium aspiration, performing BVM ventilation without prior intratracheal suctioning may worsen the resulting pneumonitis (see Chapter 37). In the case of a newborn with a congenital diaphragmatic hernia, administering positive pressure ventilation with a BVM circuit may cause air entry into the herniated segment of stomach, potentially resulting in intrathoracic tamponade and eventually cardiovascular collapse (see Chapter 39). The physiology in such circumstances is similar to that of a tension pneumothorax.
TABLE 14.1 Equipment for BVM Ventilation | |
|---|---|
|
Equipment
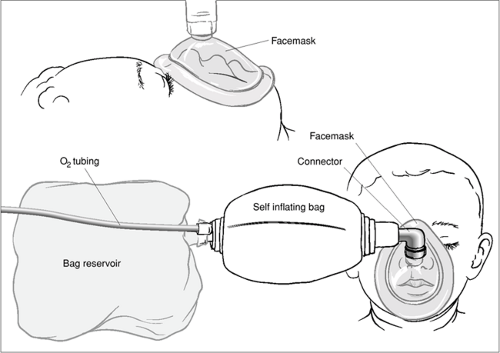
Equipment and materials used to perform BVM ventilation are listed in Table 14.1. Figure 14.8 shows a typical self-inflating BVM circuit. Appropriate sizes should be
selected carefully, as use of the proper equipment will often determine the success or failure of the procedure. Because this is often a process of trial and error, the operator should not be reluctant to change equipment during BVM ventilation should this prove necessary. As with most pediatric procedures, an array of equipment sizes should be available for all age groups (Fig. 14.9). In selecting the proper face mask, the operator should choose the smallest size that completely covers the patient’s nose and mouth (Fig. 14.10). A mask that is too large may extend below the mandible, making it difficult or impossible to establish a good seal. A large mask may also be inadvertently positioned over the eyes. Compression of the eyes can cause ocular injury or produce bradycardia as a result of vagal stimulation. Conversely, a small mask will not cover the entire mouth, causing an air leak when positive pressure ventilation is administered. It is often necessary to try more than one mask size before finding the proper fit.
selected carefully, as use of the proper equipment will often determine the success or failure of the procedure. Because this is often a process of trial and error, the operator should not be reluctant to change equipment during BVM ventilation should this prove necessary. As with most pediatric procedures, an array of equipment sizes should be available for all age groups (Fig. 14.9). In selecting the proper face mask, the operator should choose the smallest size that completely covers the patient’s nose and mouth (Fig. 14.10). A mask that is too large may extend below the mandible, making it difficult or impossible to establish a good seal. A large mask may also be inadvertently positioned over the eyes. Compression of the eyes can cause ocular injury or produce bradycardia as a result of vagal stimulation. Conversely, a small mask will not cover the entire mouth, causing an air leak when positive pressure ventilation is administered. It is often necessary to try more than one mask size before finding the proper fit.
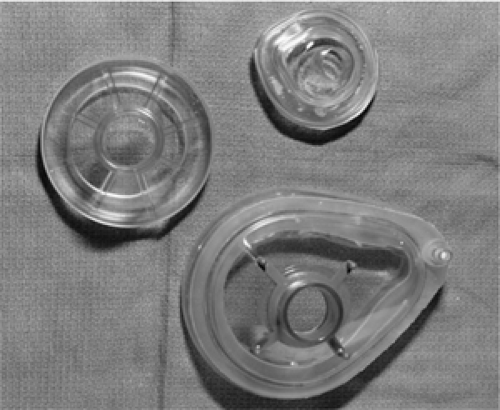 Figure 14.9 Face masks of various sizes and shapes. Premature infant through adult sizes should be available.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|