Bacterial Infections of the Central Nervous System
Thomas S. Murray and Robert S. Baltimore
BACTERIAL MENINGITIS
Meningitis, an infection of the subarachnoid space and leptomeninges caused by a variety of pathogenic organisms, continues to be an important source of mortality and morbidity. Despite the introduction of new vaccines that prevent the most severe causes, bacterial, or purulent, meningitis remains the most important form in the United States in terms of incidence, sequelae, and ultimate loss of productive life. Aseptic meningitis, usually caused by viruses, especially enteroviruses (see Chapter 306) is more common; however, significant sequelae are uncommon and the disease is usually self-limited. Granulomatous meningitis, caused either by M tuberculosis or fungi, is a major cause of neurologic injury and death in the developing world (See Chapter 269).
 EPIDEMIOLOGY
EPIDEMIOLOGY
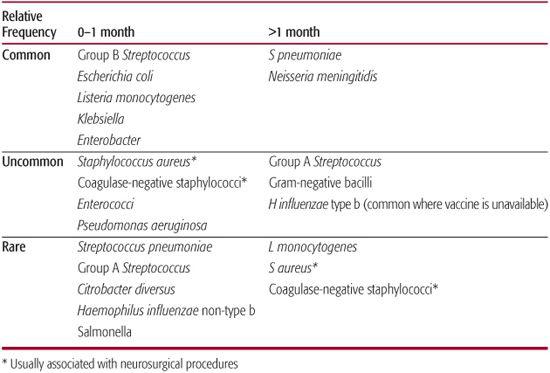
The first month after birth represents the period of highest attack rate for meningitis, with likely pathogens including group B streptococci (Streptococcus agalactiae), Escherichia coli, other gram-negative enteric organisms, and less commonly, Listeria monocytogenes (Table 231-1). Beyond the neonatal period, the most important pathogens are Streptococcus pneumoniae and Neisseria meningitidis. Formerly, Haemophilus influenzae type b (Hib) was the most common pathogen causing meningitis in infants and children, but the incidence has been reduced substantially by immunization with conjugate vaccines in developed countries.1,2 Recent studies of conjugate pneumococcal vaccine, introduced in the United States in 2000, demonstrate that it is effective in preventing pneumococcal meningitis.3 Similarly, the meningococcal polysaccharide vaccine introduced in the early 1970s has been effective in reducing meningococcal meningitis. Thus, the incidence of infection and prevailing predominant causative organisms varies depending upon the immunization status of the population.
Host factors that predispose to increased susceptibility to encapsulated organisms such as S pneumoniae increase the risk of meningitis. Neisseria infections including N meningitidis occur with increased frequency in persons with deficiencies of the terminal components of complement. Polymorphisms in toll-like receptors, integral parts of the innate immune response, have also been associated with increased risk for N meningitidis invasive disease.
The emergence of bacteria resistant to commonly prescribed antibiotics has changed empiric treatment of presumed bacterial meningitis. In the past decade, S pneumoniae with reduced susceptibility to penicillins and cephalosporins have been identified in virtually all parts of the world and, in some areas, may compromise the utility of those drugs for empiric therapy. The 7-valent pneumococcal conjugate vaccine currently available in the United States is directed at several strains associated with increased antibiotic resistance. Since its introduction, the number of strains causing invasive disease that are resistant to penicillin has declined. According to the Centers for Disease Control and Prevention, in 2002 34% of invasive infections due to S pneumoniae were caused by pneumococci nonsusceptible to at least one drug, and 17% were due to a strain nonsusceptible to 3 or more drugs. Of children who received the pneumococcal conjugate, 18.4% of those with invasive pneumococcal disease had strains intermediately susceptible to penicillin, and 19.1% had strains with high-level resistance to penicillin. More recently, a nonvaccine serotype (19A) resistant to multiple antibiotics has emerged as a cause of invasive disease.3,4 Increased antibiotic resistance has also been seen with gram-negative enteric organisms such as E coli. However, S agalactiae and N meningitidis have generally remained susceptible to penicillin and the third-generation cephalosporins. The clinician must be aware of emerging patterns of resistance within the community and in the hospital setting for patients who develop symptoms suspicious for invasive bacterial disease, including meningitis.
Table 231–1. Bacterial Causes of Meningitis
 PATHOGENESIS
PATHOGENESIS
The most common progression of infection in children with bacterial meningitis is hematogenous spread from the nasopharynx and bacterial entry into the subarachnoid space where bacterial growth occurs freely because the CSF contains few fixed or circulating scavenger cells to remove bacteria and has poor opsonic and bactericidal capability so there is not a rapid, cellular or humoral immune response.5 It may also occur as a direct extension from a contiguous focus or as a result of congenital, traumatic, or surgical disruption of normal anatomic barriers. Examples of such disruption include basilar skull fractures, placement of cerebrospinal fluid (CSF) shunts, and congenital dermal sinuses along the craniospinal axis.5
Many of the neurologic sequelae of bacterial meningitis are a consequence of altered physiology due to the host’s inflammatory response to the infecting organism (eFig. 231.1  ).6 In the subarachnoid space, components of the surface of the multiplying bacteria (lipopolysaccharide, lipo-oligosaccharide, teichoic acid) stimulate generation of proinflammatory cytokines (tumor necrosis factor [TNF]-α, inter-leukin [IL]-1β, IL-6, platelet activating factor [PAF], and others). These, in turn, increase adhesion of leukocytes to cerebral vascular endothelium, promoting increased blood-brain barrier permeability and migration of leukocytes into the subarachnoid space. White blood cell and endothelium-derived reactive oxygen species, and perhaps nitric oxide, then participate in altering cerebrovascular reactivity. Cerebral edema associated with meningitis represents a combination of vasogenic, cytotoxic, and intersititial edema. Cerebral perfusion is reduced in meningitis in approximately 30% of children in whom brain blood-flow studies have been performed. Cerebral edema not only contributes to reduced cerebral perfusion pressure but may also cause cerebral herniation due to increased intracranial pressure.5,6
).6 In the subarachnoid space, components of the surface of the multiplying bacteria (lipopolysaccharide, lipo-oligosaccharide, teichoic acid) stimulate generation of proinflammatory cytokines (tumor necrosis factor [TNF]-α, inter-leukin [IL]-1β, IL-6, platelet activating factor [PAF], and others). These, in turn, increase adhesion of leukocytes to cerebral vascular endothelium, promoting increased blood-brain barrier permeability and migration of leukocytes into the subarachnoid space. White blood cell and endothelium-derived reactive oxygen species, and perhaps nitric oxide, then participate in altering cerebrovascular reactivity. Cerebral edema associated with meningitis represents a combination of vasogenic, cytotoxic, and intersititial edema. Cerebral perfusion is reduced in meningitis in approximately 30% of children in whom brain blood-flow studies have been performed. Cerebral edema not only contributes to reduced cerebral perfusion pressure but may also cause cerebral herniation due to increased intracranial pressure.5,6
Direct cytotoxic neuronal injury, frequently found in postmortem studies, is likely caused by reactive oxygen and nitrogen species (oxygen radical, nitric oxide, peroxynitrite, hydroxyl radical), excitatory amino acids, caspases, and matrix metalloproteinases (MMPs). Experimental animal studies demonstrate improved neuronal survival when specific inhibitors of these compounds are used.7
Abnormalities of brain metabolism include hypoglycorrhachia and CSF lactic acidosis. Low CSF glucose levels occur by impaired glucose transport across the blood-brain barrier and possibly by increased cerebral glucose utilization. CSF lactic acidosis indicates anaerobic glucose utilization within the central nervous system. 
 CLINICAL MANIFESTATIONS
CLINICAL MANIFESTATIONS
The classic triad of symptoms in meningitis includes fever, headache, and stiff neck. However, in children under 2 years of age, and especially in young infants, stiff neck or other signs of meningeal irritation may be absent. Alteration of level of consciousness is usual, occurring in up to 90% of patients. The majority present with irritability, lethargy, or confusion, and 10% to 15% present in coma, a very poor prognostic sign. Physical examination of older children may reveal typical signs of meningeal irritation—stiff neck and positive Kernig and Brudzinski signs. Infants often have a bulging fontanel. Cranial nerve abnormalities, particularly of the sixth cranial nerve, may be the consequence of increased intracranial pressure or of inflammation in the subarachnoid space. Focal neurologic abnormalities are uncommon early in the disease, but when present may be indicators of cerebral infarct.1
Systemic signs may also be present in children with meningitis. The most common occur in the setting of meningococcal disease. The rash in meningococcal sepsis evolves from a transient erythematous, macular eruption to the presence of petechiae, ecchymoses, and purpura. Approximately 50% of these patients will have concurrent meningitis. Meningitis due to N meningitidis and gram-negative rod organisms frequently results in hypotension due to the systemic effects of endotoxin. Most other causes of meningitis, including those due to S pneumoniae, Hib, and L monocyto-genes, are usually not accompanied by endotoxic shock. Hypotension in the setting of disease caused by these other organisms is most commonly the result of volume depletion or purpura fulminans. Purpura fulminans is a condition associated with overwhelming infections and includes rapidly evolving disseminated intravascular coagulation (DIC), fever, chills, and ecchymotic skin lesions that may ulcerate and progress to peripheral gangrene, shock, and death.1
 DIAGNOSIS
DIAGNOSIS
Once the diagnosis of meningitis is suspected, immediate examination of the CSF is indicated. A specific reason to delay lumbar puncture is if there is a strong suspicion of an intracranial mass lesion. Worrisome findings include papilledema or a history of an indolent process with focal neurologic findings. In those instances, lumbar puncture can be delayed until a mass can be excluded by cranial computerized tomography (CT) or magnetic resonance (MR) scanning. CSF abnormalities include elevated numbers of white blood cells, elevated protein concentration, hypoglycorrhachia, elevated opening pressure, and organisms observed on Gram stain. Pleocytosis is typical in bacterial meningitis, with CSF white blood cell (WBC) concentrations in the range of 100 to 10,000 cells/μL, although occasionally early in the disease, the WBC concentration may be normal or slightly elevated. Polymorphonuclear cells predominate and usually account for more than 90% of the total. Very high WBC concentrations (greater than 50,000/μL) raise the possibility of an intracranial abscess that has ruptured into the ventricles.1
Hypoglycorrhachia is commonly found in bacterial meningitis, with CSF glucose usually less than 50% of simultaneous serum glucose. Other causes of hypoglycorrhachia include tuberculous and fungal meningitis, subarachnoid hemorrhage, and carcinomatous meningitis. Cerebrospinal fluid protein concentration is usually elevated, in the range of 100 to 500 mg/dL, but as elevated protein reflects an alteration in the blood-brain barrier, it is not, by itself, diagnostic of bacterial infection.
In contrast to bacterial meningitis, CSF from patients with aseptic (including viral) meningitis is typically characterized by lower WBC counts and a glucose concentration near or within the normal range. Although the percentage of neutrophils may be variable, it is usually much lower in aseptic meningitis where a lymphocyte predominance is more common. CSF protein concentration may be normal or elevated in patients with meningitis caused by enterovirus or M tuberculosis.
The Gram stain is positive in more than 90%, and the CSF culture is positive in 70% to 90% of patients with untreated hematogenous bacterial meningitis.1
Prior treatment with oral antibiotics substantially reduces the yield of CSF bacterial cultures. In this setting, the use of antigen-detection methods such as latex particle agglutination may help in etiologic identification. More recently, molecular diagnostic methods, including polymerase chain reaction (PCR) of a portion of the bacterial gene encoding the 16S ribosome, have been successful in the early diagnosis and speciation of bacterial meningitis (see Future Directions). One of these methods, when performed in the first 48 to 72 hours after oral antibiotics are given, will identify the pathogen in approximately 90% of patients.8,9
In addition to culture and Gram stain of the CSF, blood culture may be useful in specific etio-logic diagnosis. Blood cultures are positive in 40% to 75% of cases of bacterial meningitis, depending on the pathogen, and should be performed routinely.
Studies other than those aimed at making an etiologic diagnosis are indicated as well. Peripheral-blood WBC and differential are useful in assessing the likelihood of a serious bacterial infection, and leukopenia may be a poor prognostic sign, indicating failure of the inflammatory response, particularly in the settings of meningococcal and pneumococcal disease. Similarly, prolonged blood coagulation studies in conjunction with thrombocytopenia may indicate DIC, which is often present with serious gram-negative infection. Serum and urine electrolytes and osmolality should be measured routinely to look for the syndrome of inappropriate antidiuretic hormone (SIADH) secretion, marked by hyponatremia in association with increased urine osmolality and increased urine concentration of sodium. Additional studies, such as roentgenographic examinations, are rarely needed in the diagnosis of meningitis; however, they may be useful in identifying coexisting complicating factors. Cranial CT may indicate cerebral edema early in the infection, and later may show subdural effusion or subdural empyema.1
 TREATMENT
TREATMENT
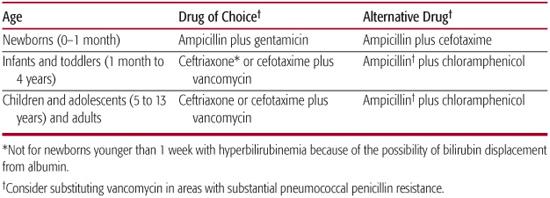
Effective treatment of meningitis depends on early aggressive supportive therapy and selection of empiric antimicrobials appropriate for the likely pathogens. The principles of antimicrobial therapy of meningitis include selection of an antibiotic that is bactericidal against the suspected pathogen and that achieves a concentration in CSF at least 10 times the minimal bactericidal concentration for the organism, as this is the concentration that has been shown in animal studies to correlate with most effective sterilization of CSF.10 Suggested choices for empiric therapy are listed in Table 231-2 and should be based on the likely pathogens for age group, known exposures, and any unusual risk factors for the patient. Definitive antibiotic therapy depends on the antibiotic susceptibility of the organism. N meningitidis is susceptible to penicillin but cefotaxime or ceftriaxone can be used. For patients who must avoid all beta-lactam antibiotics, chloramphenicol would be an appropriate choice. Hib are uniformly susceptible to ceftotaxime or ceftriaxone, but the majority of isolates are susceptible to ampicillin, which is the drug of choice if the isolate is susceptible. Doses of antibiotics used to treat the more common causes of bacterial meningitis in children are shown in eTable 231.1  .
.
The numbers of pneumococcal clinical isolates with high-level resistance to third-generation cephalosporins have been increasing, so empiric therapy with vancomycin is warranted when pneumococcal meningitis is suspected. Duration of antibiotic therapy is 14 to 21 days for neonatal meningitis caused by group B streptococci, and a minimum of 21 days for gram-negative enteric organisms. For meningitis in older infants or children, treatment should be 7 days for N meningitidis, 7 to 10 days for Hib, and 10 to 14 days for S pneumoniae.11
Regarding the possible benefits of adjunctive early corticosteroid treatment, despite numerous studies, there have been inconsistent results in children with bacterial meningitis (not caused by Hib). Dexamethasone, 0.15 mg/kg per dose, administered prior to or concurrently with the start of antibiotic treatment and continued every 6 hours for 2 to 4 days is the regimen used in published studies. Some data suggest steroid administration improves mortality in adults with pneumococcal meningitis, but there is insufficient literature to support this as a blanket recommendation for children.12-14
There are supportive measures that address the consequences of serious intracranial pathology. Patients who are comatose or who have impaired gag reflex should have their stomach contents emptied, and intubation should be considered to protect the airway. Hypoxia should be treated with supplemental oxygen. Hypoventilation is particularly worrisome in these patients because elevated PACO2 may cause cerebral vasodilatation and potentiate increased intracranial pressure. Hypercarbia should be considered as another indication for intubation and assisted ventilation.
Fluid management is critically important in patients with meningitis. SIADH occurs in approximately 30% of patients with bacterial meningitis, and warrants fluid restriction after restoration of normal blood volume. However, a clinical study has documented the importance of maintaining an adequate cerebral perfusion pressure in this disease. Inappropriate fluid restriction may result in volume depletion, leading to inadequate circulating volume if carried to extremes. If SIADH is present, fluids should be limited to replacement of insensible losses plus urine output (generally, approximately two thirds of maintenance requirement) until ADH excess resolves. If SIADH is not present, fluids should be administered in an amount appropriate to maintenance requirements plus intercurrent losses, and electrolytes should be carefully monitored.15
Therapy of increased intracranial pressure must be directed at maintaining an adequate degree of cerebral perfusion pressure as in other conditions complicated by intracranial hypertension (see Chapter 111).
 OUTCOME
OUTCOME
Mortality rates in bacterial meningitis vary considerably depending on the age of the patient and the pathogen. Individuals with meningococcal meningitis without overwhelming meningococcemia have a fatality rate of 12%, whereas newborns with gram-negative meningitis succumb up to 70% of the time. Death rates from Hib and S pneumoniae are approximately 3% and 6%, respectively, at children’s hospitals in developed countries.
Morbid sequelae occur in approximately 30% of survivors, but there is also an age and pathogen predilection, with the greatest incidence of sequelae occurring among the very young and in those infected with either gram-negative bacteria or S pneumoniae. In addition to depressed mental status at the time of presentation, other poor prognostic factors include the presence of comorbid conditions, leukopenia, and severe hypoglycorrhachia. The most common neurologic sequelae include deafness in 3% to 25% of patients; cranial nerve palsies in 2% to 7%; and severe injury, such as hemiparesis or global brain injury, in 1% to 2% of patients. More than 50% of patients with neurologic sequelae at discharge from hospital will improve with time, and recent advances in cochlear implant therapy may provide hope for the child with hearing loss.1
 PREVENTION
PREVENTION
Prevention of meningitis currently takes two forms: chemoprophylaxis for susceptible individuals known to be exposed to an index patient and active immunization. Chemoprophylaxis is currently indicated for preventing secondary meningitis due to Hib and N meningitidis (see Chapters 263 and 275). Active immunization with conjugate vaccine against Hib has resulted in a dramatic reduction in invasive disease, with a >95% reduction in meningitis caused by that organism. The introduction of the conjugate pneumococcal vaccine has also reduced rates of invasive pneumococcal disease such as meningitis, including in high-risk communities, such as Native American communities. A quadrivalent conjugate meningococcal vaccine has become available against groups A, C, Y, and W-135 N meningitidis. It is indicated for children over 2 years of age who are considered to be at increased risk of meningococcal infection and for all young adolescents. 
Table 231–2. Empiric Antimicrobial Therapy for Presumed Bacterial Meningitis
BRAIN ABSCESS
Brain abscess is relatively uncommon yet remains a serious disease. Early diagnosis with neuroradiologic imaging, rapid neurosurgical intervention (including stereotactic brain biopsy and aspiration), and the initiation of broad spectrum antimicrobial therapy treatment, to cover both anaerobic and aerobic bacteria, provide the best opportunity for a favorable outcome. Nevertheless, because there are no prospective controlled or comparative trials, management relies largely on retrospective studies and clinical experience.
 EPIDEMIOLOGY
EPIDEMIOLOGY
The incidence of brain abscess is difficult to evaluate but is probably 2 to 3 per 10,000 general hospital admissions. A preponderance of male patients has been noted in most large series. Brain abscess is rare in infants less than 1 year of age, but the incidence rises rapidly thereafter, and nearly one third of all brain abscesses occur in the pediatric age group. The low incidence of brain abscesses in infants is related to their lack of well-formed frontal or mastoid sinuses.16,17
One risk factor for the development of brain abscesses is cyanosis, caused either by congenital heart disease or by pulmonary arteriovenous shunting. Postmortem studies have indicated that brain abscesses are found in 0.4% of patients dying from all causes, whereas in patients with congenital heart disease, the incidence may be as high as 6%. The incidence of brain abscess in all children with congenital cyanotic heart disease is 2% to 3%. Children with right-to-left intracardiac shunting are deprived of the phagocytic filtering action of the pulmonary capillary bed, and in these children, the cerebral circulation is subject to recurrent bacteremia. 
Immunosuppressed children are also at risk for brain abscess. Abscesses in this group are more likely to be multiple, the children are usually debilitated, and the abscesses are more likely to contain multiple species, including fungi.
 PATHOPHYSIOLOGY
PATHOPHYSIOLOGY
Most abscesses start at the gray-white matter junction. Encapsulation proceeds more rapidly and profusely on the cortical side than on the side facing the white matter. With abscesses in paraventricular sites, rupture into the ventricle may occur at any time, an event that carries a very high mortality.
Brain abscess can arise secondary to infection elsewhere in the body or can be caused by penetrating injury or a neurosurgical procedure. Extension and spread of infection via the bloodstream through venous channels to the brain from paranasal, mastoid, or the inner ear has been reported as the most common cause of brain abscess in many series. However, the incidence of infection from otorhinologic sources has fallen markedly since the introduction of antibiotics. Meta-static abscesses usually originate in either the heart or the lungs, although osteomyelitis, renal infections, and skin abscess (including the scalp) can be the primary sources. Anatomic anomalies that predispose children to brain infections include right to left cardiac shunting, pulmonary arteriovenous malformations, and congenital dermoids or dermal sinus tracts.
 BACTERIOLOGY
BACTERIOLOGY
Material from abscess cavities must be cultured immediately, both aerobically and anaerobically. Anaerobic bacteria, including Bacteroides fragilis, play a prominent role in brain abscess formation, accounting for almost 80% of nontraumatic brain abscesses in some series. The most common isolate is Streptococcus with a predominance of S milleri in the viridans group. Abscesses resulting from trauma most commonly culture Staphylococcus aureus. Other causative organisms are other Bacteroides species, hemolytic streptococci, Proteus species, H influenzae, E coli, and, rarely, Cryptococcus, Nocardia, Aspergillus, or Corynebacterium species. Still, more than 10% of cultures will be sterile, using the best contemporary culture techniques.
The bacterial spectrum in neonates with brain abscesses differs from that in older infants and children. Nearly half the reported infections in infants less than 3 months of age have been caused by gram-negative organisms including E coli. Citrobacter, Proteus, and Salmonella.19
 CLINICAL PRESENTATION
CLINICAL PRESENTATION
The initial symptoms of brain abscess are more likely to be related to its intracranial mass effect than to the infectious nature of the illness. Lethargy, anorexia, and vomiting are noted, and older children will complain of headache. Occasionally, there may be a distinct period of cerebritis or meningitis preceding abscess formation. Focal or grand mal seizures may be the first indication of cerebral involvement and may lead to discovery of previously unnoticed neurologic deficits. Fever may be quite mild; almost half of these children are afebrile on hospital admission, even though there is a history of recent febrile illness. Brain abscesses are usually rapidly progressive; the duration of symptoms is often less than a week and seldom more than a month. Specific neurologic deficits will depend on the area of involvement and may include hemiparesis, sensory impairment, and visual field abnormalities. Posterior fossa abscess will cause ataxia, dysmetria, and cranial nerve palsies.16,17
 DIAGNOSIS
DIAGNOSIS
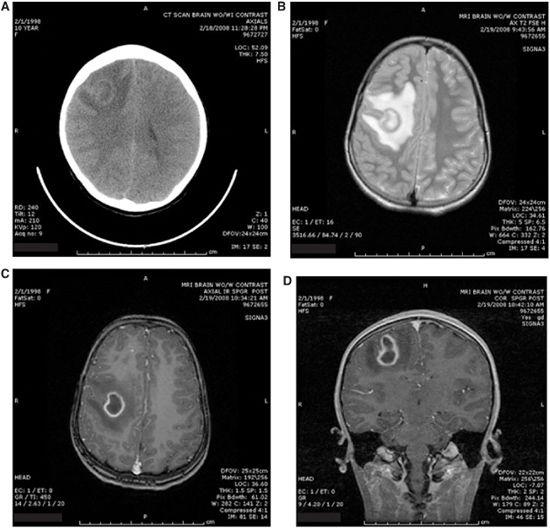
Both CT scanning and MR imaging are useful to diagnose and follow intracranial abscess. MR imaging will often provide better resolution of the abscess and surrounding edema when compared with CT, but CT imaging is usually more rapidly available in the emergency department (Fig. 231-1). CT and MR scans should be performed with and without contrast enhancement. Following contrast injection, scans performed both immediately and after delay of 30 to 60 minutes will allow for more precise staging of the abscess. Multiple lesions, which cause high mortality among patients with brain abscesses, are easily diagnosed. Dosages of steroids, antibiotics, and even mannitol may be adjusted according to the changes observed over time.18,20
The results of routine hematology laboratory studies may be normal or may reveal moderate leukocytosis and an elevated erythrocyte sedimentation rate.
Many reports have stressed the danger of lumbar puncture in the presence of abscess. If there are indications of increased intracranial pressure or shifting of intracranial structures, lumbar puncture is contraindicated. The information obtained from the CSF in patients with brain abscess is usually nonspecific; often the causative organisms cannot be demonstrated, and lumbar puncture may introduce a potentially fatal risk.
 TREATMENT
TREATMENT
The initiation of appropriate therapy requires a rapid assessment of the patient’s clinical status and the expertise of an experienced neurosurgeon. For patients who display clinical or radiographic evidence of increased intracranial pressure, initial therapy is aimed at decreasing the intracerebral pressure and mass effect and includes steroids. Periabscess edema derives from both the local mass and the stasis effect and inflammation response that causes a diffuse increase in brain water. Steroids in large doses decrease the endothelial permeability of vessels in the inflammatory area and reduce the excessive edema. Although the restorative effect of steroids on the blood-brain barrier may reduce antibiotic activity in the infected region, this effect is usually of lesser concern than the need to reduce increased intracranial pressure. Patients who do not have evidence of increased intracranial pressure or mass effect may not require steroids or immediate neurosurgical intervention but must have frequent neurological exams to check for changes in the patient’s status.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


