Introduction. Bacterial sepsis and meningitis continue to be major causes of morbidity and mortality in newborns, particularly in premature infants. Although improvements in neonatal intensive care have decreased the impact of early-onset sepsis (EOS) in term infants, preterm infants remain at high risk for both EOS and its sequelae. Very low birth weight (VLBW) infants are also at risk for late-onset (hospital-acquired) sepsis. Neonatal survivors of sepsis can have severe neurologic sequelae due to central nervous system (CNS) infection, as well as from secondary hypoxemia resulting from septic shock, persistent pulmonary hypertension, and severe parenchymal lung disease.
Epidemiology of EOS. The overall incidence of EOS has decreased significantly since the Centers for Disease Control and Prevention (CDC) first published recommendations for intrapartum antibiotic prophylaxis (IAP) against group B Streptococcus (GBS) in 1996. Studies conducted afterwards showed the overall incidence of EOS to be approximately 1 to 2 cases per 1,000 live births. The incidence is twice as high among moderately premature infants, and highest among VLBW (< 1,500 g) infants with recent reports ranging from 15 to 23 cases per 1,000 VLBW births.
Risk factors for EOS. Maternal and infant characteristics associated with the development of EOS have been most rigorously studied with respect to GBS EOS. Maternal factors predictive of GBS disease include documented maternal GBS colonization, intrapartum fever (>38°C) and other signs of chorioamnionitis, and prolonged rupture of membranes (ROM) (>18 hours). Neonatal risk factors include prematurity (<37 weeks’ gestation) and low birth weight (LBW) (<2,500 g).
Clinical presentation of EOS. Early-onset disease can manifest as asymptomatic bacteremia, generalized sepsis, pneumonia, and/or meningitis. The clinical signs of EOS are usually apparent in the first hours of life; 90% of infants are symptomatic by 24 hours of age. Respiratory distress is the most common presenting symptom. Respiratory symptoms can range in severity from mild tachypnea and grunting, with or without a supplemental oxygen requirement, to respiratory failure. Persistent pulmonary hypertension of the newborn (PPHN) can also accompany sepsis. Other less specific signs of sepsis include irritability, lethargy, temperature instability, poor perfusion, and hypotension. Disseminated intravascular coagulation (DIC) with purpura and petechiae can occur in more severe septic shock. Gastrointestinal (GI) symptoms can include poor feeding, vomiting, and ileus. Meningitis may present with seizure activity, apnea, and depressed
sensorium, but may complicate sepsis without specific neurologic symptoms, underscoring the importance of the lumbar puncture (LP) in the evaluation of sepsis.
Other diagnoses to be considered in the immediate newborn period in the infant with signs of sepsis include transient tachypnea of the newborn, meconium aspiration syndrome, intracranial hemorrhage, congenital viral disease, and congenital cyanotic heart disease. In infants presenting at more than 24 hours of age, closure of the ductus arteriosus in the setting of a ductaldependent cardiac anomaly (such as critical coarctation of the aorta or hypoplastic left heart syndrome) can mimic sepsis. Other diagnoses that should be considered in the infant presenting beyond the first few hours of life with a sepsis-like picture include bowel obstruction, necrotizing enterocolitis (NEC), and inborn errors of metabolism.
Evaluation of the symptomatic infant for EOS. Laboratory evaluation of the symptomatic infant suspected of EOS includes at minimum a complete blood count (CBC) with differential and blood culture. Other laboratory abnormalities can include hyperglycemia and metabolic acidosis. Thrombocytopenia as well as evidence of DIC (elevated prothrombin time [PT], partial thromboplastin time [PTT], and international normalized ratio [INR]; decreased fibrinogen) can be found in more severely ill infants. For infants with a strong clinical suspicion of sepsis, a LP for cerebrospinal fluid (CSF) cell count, protein and glucose concentration, Gram stain, and culture should be performed before the administration of antibiotics if the infant is clinically stable. The LP may be deferred until after the institution of antibiotic therapy if the infant is clinically unstable, or if later culture results or clinical course demonstrate that sepsis was present.
Infants with respiratory symptoms should have a chest radiograph as well as other indicated evaluation such as arterial blood gas measurement. Radiographic abnormalities caused by retained fetal lung fluid or atelectasis usually resolve within 48 hours. Neonatal pneumonia will present with persistent focal or diffuse radiographic abnormalities and variable degrees of respiratory distress. Neonatal pneumonia (particularly that caused by GBS) can be accompanied by primary or secondary surfactant deficiency.
Treatment of EOS. Empiric antibiotic therapy includes a broad coverage for organisms known to cause EOS, usually a β-lactam antibiotic and an aminoglycoside. In our institutions, we use ampicillin and gentamicin as initial therapy. We add a third-generation cephalosporin (cefotaxime or ceftazidime) to the empiric treatment of critically ill infants for whom there is a strong clinical suspicion for sepsis to optimize therapy for ampicillin-resistant, enteric gram-negative organisms, primarily ampicillin-resistant Escherichia coli. (See Table 49.1 for treatment recommendations.) Supportive treatments for sepsis include the use of mechanical ventilation, exogenous surfactant therapy for pneumonia and respiratory distress syndrome (RDS), volume and pressor support for hypotension and poor perfusion, sodium bicarbonate for metabolic acidosis, and anticonvulsants for seizures. Echocardiography may be of benefit in the severely ill, cyanotic infant to determine if significant pulmonary hypertension or cardiac failure is present. Infants born at ≥34 weeks with symptomatic pulmonary hypertension may benefit from treatment with inhaled nitric oxide (iNO). Extracorporeal membrane oxygenation (ECMO) can be offered to infants ≥34 weeks if respiratory and circulatory failure occurs despite all conventional measures of intensive care. ECMO is not generally available to infants less than 34 weeks’ gestation.
Table 49.1 Suggested Antibiotic Regimens for Sepsis and Meningitis*
Organism
Antibiotic
Bacteremia
Meningitis
GBS
Ampicillin or penicillin G
10 d
14-21 d
E. coli
Cefotaxime or ampicillin and gentamicin
10-14 d
21 d
CONS
Vancomycin
7 d
14 d
Klebsiella, Serratia†
Cefotaxime or meropenem and gentamicin
10-14 d
21 d
Enterobacter, Citrobacter‡
Cefepime or meropenem and gentamicin
10-14 d
21 d
Enterococcus§
Ampicillin or vancomycin and gentamicin
10 d
21 d
Listeria
Ampicillin and gentamicin
10-14 d
14-21 d
Pseudomonas
Ceftazidime or piperacillin/tazobactam and gentamicin or tobramycin
14 d
21 d
S. aureus¶
Nafcillin
10-14 d
21 d
MRSA
Vancomycin
10-14 d
21 d
GBS = group B Streptococcus; CONS = coagulase-negative staphylococci; MRSA = Methicillin-resistant Staphylococcus aureus.
*All treatment courses are counted from the first documented negative blood culture and assume that that antibiotic sensitivity data are available for the organisms. In lateonset infections, all treatment courses assume central catheters have been removed. With CONS infections, the clinician may choose to retain the catheter during antibiotic treatment, but if repeated cultures remain positive, the catheters must be removed. Many infectious disease specialists recommend repeat lumbar punctures at the completion of therapy for meningitis to ensure eradication of the infection.
† †The spread of plasmid-borne extended-spectrum beta-lactamases (ESBL) among enteric pathogens such as E. coli, Klebsiella, and Serratia is an increasing clinical problem. Recent literature suggests that ESBL-containing organisms can be effectively treated with cefepime or meropenem. Reports of carbapenemase-producing organisms are of concern and infection with these requires consultation with an infectious disease specialist.
‡ ‡Enterobacter and Citrobacter species have inducible, chromosomally-encoded cephalosporinases. Cephalosporins other than the fourth generation cefepime should not be used to treat infections with these organisms even if initial in vitro antibiotic sensitivity data suggest sensitivity to third-generation cephalosporins such as cefotaxime. There are some reports in the literature of cefepime-resistant Enterobacter.
§ §Enterococci are resistant to all cephalosporins. Ampicillin-resistant strains of enterococci are common in hospitals, and require treatment with vancomycin. Treatment of vancomycin resistant strains (VRE) requires consultation with an infectious disease specialist.
¶ ¶Uncomplicated methicillin-sensitive S. aureus and MRSA bacteremias may be treated for only 10 days if central catheters have been removed. Persistent bacteremias can require treatment for 3 to 4 weeks. Bacteremias complicated by deep infections such as osteomyelitis or infectious arthritis often require surgical drainage and treatment for up to 6 weeks. The use of additional agents such as linezolid, daptomycin and rifampin to eradicate persistent S. aureus infection; or to treat vancomycin-intermediately susceptible (VISA) and resistant (VRSA) strains requires consultation with an infectious disease specialist.
A variety of adjunctive immunotherapies for sepsis have been trialed since the 1980s to address deficits in immunoglobulin and neutrophil number and function. Double-volume exchange transfusions, granulocyte infusions, the administration of intravenous immunoglobulin (IVIG), and treatment with granulocyte-colony stimulating factor (G-CSF) and granulocyte macrophage-colony stimulating factor (GM-CSF) have all been investigated with variable results.
Double-volume exchange transfusion and granulocyte infusion. Several experimental approaches have been taken to replete neutrophils in neutropenic septic infants: (i) double-volume exchange transfusion with fresh whole blood, (ii) infusion of fresh buffy-coat preparations, or (iii) infusion of granulocytes collected by leukopheresis. Two small, randomized controlled trials of exchange transfusion with whole blood in infants with (largely gram-negative) sepsis were published in the 1990s. Both reported a 50% reduction in mortality of the infants undergoing exchange, and demonstrated increases in neutrophil number, improvement in neutrophil function, and increases in immunoglobulin concentration in the exchanged infants. A Cochrane review of four small trials of granulocyte transfusion in neutropenic neonates with sepsis concluded that there is insufficient evidence of survival benefit with this therapy. Both whole blood exchange transfusion and granulocyte infusion present significant risks, including graft-versus-host disease, blood-group sensitization, and transmission of infections such as cytomegalovirus (CMV), HIV, and viral hepatitis. In addition, the emergent availability of these blood products (especially leukopheresed granulocytes) is limited in most centers. We do not currently use either of these treatments in the treatment of early- or late-onset sepsis.
IVIG. The use of IVIG in the acute treatment of neonatal sepsis is controversial. It is likely that any efficacy of IVIG would be highest in EOS, which in the United States is largely due to the encapsulated organisms such as GBS and E. coli K1, and in premature infants, who are most likely to have inadequate immunoglobulin reserves. IVIG trials reported to date have been conducted in several different countries, using different dosing regimens and/or immunoglobulin preparations. Meta-analysis of randomized trials of the use of IVIG in the acute treatment of suspected or proven neonatal sepsis shows a decrease in mortality of borderline significance. IVIG is expensive and has potential infectious risks, and based on the current marginal evidence of benefit, most authorities have not endorsed the routine use of IVIG in the treatment of neonatal sepsis.
Cytokines. Recombinant G-CSF and GM-CSF have been shown to restore neutrophil levels in small studies of neutropenic growth-restricted infants, ventilator-dependent neutropenic infants born to mothers with preeclampsia, and in neutropenic infants with sepsis. A rise in the absolute neutrophil count (ANC) above 1,500/mm3 occurred in 24 to 48 hours. To date, seven randomized controlled trials of recombinant colony-stimulating factors have been reported, all enrolling small numbers of infants. Assessment of these trials is complicated by the use of different preparations, dosages, and durations of therapy, as well as variable enrollment criteria (differing gestational age ranges, presumed and culture-proven sepsis, neutropenic and non-neutropenic infants, early- and late-onset of infection). None of the trials included neurodevelopmental follow-up. These studies suggest that G-CSF may result in lower mortality among neutropenic, septic VLBW infants; but, overall, there
is currently insufficient evidence to support the routine use of these preparations in the acute treatment of neonatal sepsis.
Activated protein C (APC) and pentoxifylline. Both of these immunomodulatory preparations have been studied in adults with severe sepsis. Both are active in preventing the microvascular complications of sepsis, by promoting fibrinolyis (APC) and improving endothelial cell function (pentoxifylline), and both decrease the production of tumor necrosis factor (TNF). APC has not been studied in neonates in randomized trials. Pentoxifylline has been studied in a small number of preterm infants with late-onset sepsis with improvement in mortality. Neither medication can be recommended for use in neonates without further study.
Evaluation of the asymptomatic infant at risk for EOS. There are a number of clinical factors that place infants at risk for EOS. These factors also identify a group of asymptomatic infants who may have colonization or bacteremia that places them at risk for the development of symptomatic EOS. These infants include those born to mothers who have received inadequate intrapartum antibiotic prophylaxis (IAP) for GBS (see subsequent text) and those born to mothers with suspected chorioamnionitis. Blood cultures are the definitive determination of bacteremia. A number of laboratory tests have been evaluated for their ability to predict which of the at-risk infants will go on to develop symptomatic or cultureproven sepsis, but no single test has adequate sensitivity and specificity.
Blood culture. With advances in the development of computer-assisted, continuous-read culture systems, most blood cultures will be positive within 24 to 36 hours of incubation if organisms are present. Most institutions, including ours, empirically treat infants for sepsis for a minimum of 48 hours with the assumption that true positive cultures will turn positive within that period. At least 0.5 mL (and preferably 1 mL) of blood should be placed in most pediatric blood culture bottles. We use two culture bottles, one aerobic and one anaerobic. Certain organisms causing EOS (such as Bacteroides fragilis [B. fragilis]) will only grow under anaerobic conditions; 5% of culture-proven EOS in our institution is due to strictly anaerobic species. Additionally, GBS, Staphylococcus species, and many gram-negative organisms grow in a facultative fashion, and the use of two culture bottles increases the likelihood of detecting low-level bacteremia with these organisms.
White blood count (WBC). The WBC and differential is readily available and commonly used to evaluate both symptomatic and asymptomatic infants at risk for sepsis. Interpretation of neonatal WBC has been compromised by the relatively small size of studies used to determine normal values and by a lack of data quantifying the impact of differences mediated by gestational age, postnatal age, mode of delivery, and maternal conditions. Maternal fever, neonatal asphyxia, meconium aspiration syndrome, pneumothorax, and hemolytic disease have all been associated with neutrophilia; maternal pregnancy-induced hypertension and preeclampsia are associated with neonatal neutropenia as well as thrombocytopenia.
One finding common to all published neonatal WBC data is the “roller coaster” shape of the WBC, ANC, and immature to total neutrophil ratio (I:T) curves in the first 72 hours of life. This suggests that optimal interpretation of WBC data to predict EOS should account for the natural rise and fall in WBC during this period. A recent study supports the use of CBC
only after the first few hours of life, when placed in the proper clinical context and used as part of an algorithm to evaluate infants for sepsis risk. In this study, both the WBC and ANC were most predictive of infection when these values were low (WBC <5,000 and ANC <2,000) and when obtained at more than 4 hours of age. An elevated WBC (>20,000) was neither worrisome nor reassuring in neonates. The I:T ratio was most informative if measured beyond 1 to 4 hours after birth, with low values (<0.15) reassuring, whereas elevated values (>0.3) were weakly associated with EOS.
Although studies demonstrate that no component of the WBC is very sensitive among term and late preterm infants for the prediction of sepsis, there are little data to guide interpretation of the WBC among VLBW infants at risk for EOS. It is possible that the WBC and its components may be of more value in the VLBW infant and/or in the evaluation of late-onset infection. Finally, some centers combine WBC values in a “sepsis screen” (e.g., the use of an algorithm incorporating total WBC, I:T ratio, total band count, with or without C-reactive protein [CRP] values) to guide treatment decisions.
CRP is a nonspecific marker of inflammation or tissue necrosis. Elevations in CRP are found in bacterial sepsis and meningitis. A single determination of CRP at birth lacks both sensitivity and specificity for infection, but serial CRP determinations at birth and at 12 hours and beyond have been used to manage infants at risk for sepsis. Some centers use serial CRP measurements to determine length of antibiotic treatment for infants with culture-negative clinical sepsis. We do not use CRP measurements in the evaluation of infants at risk for sepsis.
Cytokine measurements. Advances in the understanding of the immune responses to infection and in the measurement of small peptide molecules have allowed investigation into the utility of these inflammatory molecules in predicting infection in neonates at risk. Serum levels of interleukin-6, interleukin-8, interleukin-10, interleukin-1 β, G-CSF, TNF-α, and procalcitonin, as well as measurements of inflammatory cell-surface markers such as CD64, have been variably correlated with culture-proven, clinical, and viral sepsis. The need for serial measurements and the availability of the specific assays so far limit the use of cytokine markers in diagnosing neonatal infection. In addition, most studies have been performed on infants who are symptomatic and being evaluated for sepsis. None of these have yet proven useful in predicting infection in initially well-appearing infants.
Other strategies. Urine latex particle agglutination testing for GBS remains available at some institutions, but we have given up the use of this test due to very poor predictive value. Latex particle testing of CSF for both GBS and E. coli K1 can be of use in evaluating CSF after the institution of antibiotic treatment.
LP. The use of routine LP in the evaluation of asymptomatic neonates at risk for EOS remains controversial. A retrospective review of 9,111 infants born at ≥34 weeks’ gestation from 150 neonatal intensive care unit (NICU) on whom a LP was performed found 95 cases of culture-proven meningitis. In 38% of these cases, the accompanying blood culture was sterile. Another retrospective study of CSFs taken from a population of 169,849 infants identified eight infants with culture-positive CSF, but with negative blood cultures and no CNS symptoms. In both studies, the authors concluded that the selective use of LP in the evaluation of EOS may lead to missed diagnoses of meningitis. However, in both studies, infants were not all evaluated for sepsis in the absence
of symptoms, and the subjects were drawn from large numbers of hospitals with likely disparate culture systems. Another study reviewed the results of sepsis evaluations in a population of 24,452 infants from a single institution. This study found 11 cases of meningitis, all in symptomatic infants; 10 of 11 corresponding blood cultures were positive for the same organism. No cases of meningitis were found in 3,423 asymptomatic infants evaluated with LP.
We do not perform LP’s for the evaluation of asymptomatic term infants at risk for EOS. A review of our own data from 1996 to 2009, a period during which IAP for GBS was implemented using a screening-based approach, revealed 20 cases of culture-positive meningitis from a population of over 70,000 deliveries. Only two cases occurred in term infants; both infants grew GBS from both blood and CSF cultures and both infants were symptomatic. It is our current policy to perform LPs only on (i) infants with positive blood cultures and (ii) symptomatic infants with negative blood cultures who are treated empirically for the clinical diagnosis of sepsis. Whenever clinically feasible, LPs are performed on symptomatic infants with a high suspicion for sepsis before administering antibiotics.
When LPs are performed after the administration of antibiotics, a clinical evaluation of the presence of meningitis is made, taking into account the blood culture results, the CSF cell count, protein, and glucose levels, as well as the clinical scenario. We recommend sending two separate CSF samples for cell count from the same LP in these circumstances to account for the role of possible fluctuation in CSF cell count measurements. Interpretation of CSF WBC values can be challenging. Normal CSF WBC counts in term, noninfected infants are variable, with most studies reporting a mean of <20 cells per mm3, with ranges of up to 90 cells, and widely varying levels of polymorphonuclear cells on the differential. One recent study defined “noninfected” infants by negative bacterial blood, CSF and urine cultures, and negative viral CSF culture, as well as negative enteroviral CSF polymerase chain reaction (PCR). This study reported a mean CSF WBC 7.3 (±14) per mm3 with a range of 0 to 130 cells. Another study of culture-proven, early-onset meningitis demonstrated only 80% sensitivity and specificity for CSF WBC values >20. The presence of blood in the CSF, due to subarachnoid or intraventricular hemorrhage, or to blood contamination of CSF samples by “traumatic” LPs, can yield abnormal cell counts that may be due to the presence of blood in the CSF rather than true infection. Adjustment of the WBC in traumatic LP results (those with >500 RBC per mm3) using different algorithms has not been shown to substantially improve the sensitivity and specificity of the WBC in predicting culture-confirmed meningitis.
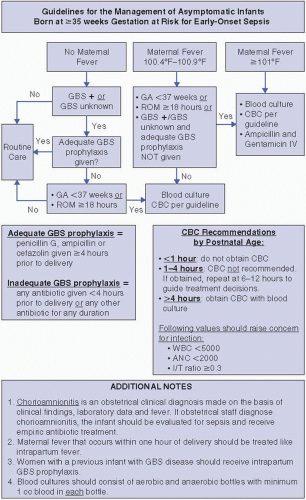
Algorithm for the evaluation of the infant born at ≥35 weeks’ gestation at risk for EOS. At the Brigham and Women’s Hospital (BWH), to ensure consistency amongst caregivers, an algorithm is used for the evaluation of asymptomatic, ≥35-week gestation infants who are at risk for developing EOS (Figure 49.1). This algorithm incorporates both the evaluation of infants based on maternal GBS colonization, and an evaluation of infants at risk for EOS due to maternal intrapartum risk factors. A total WBC of <5,000 or an I:T ratio of >0.3 is used to guide treatment decisions in the evaluation of the well-appearing infant at risk for sepsis. A single CBC determination is used in most cases to avoid multiple blood draws from otherwise asymptomatic infants; as noted previously, WBC values may have better predictive value when performed after 1 to 4 hours of age. We use a fever threshold of 100.4°F (38°C) for evaluation in accordance with CDC and other published recommendations. We take into account the impact of a clustering of risk factors for sepsis to guide treatment decisions, as well as the use of intrapartum antibiotic therapy, to guide management decisions. These guidelines are based on a delivery service for which a screening-based approach to GBS prophylaxis has been in place since 1996, and for which the vast majority of vaginal deliveries involve epidural placement (which alone can cause low-grade intrapartum fever).
Specific organisms causing EOS. The bacterial species responsible for EOS vary by locality and time period. In the United States since the 1980s, GBS has been the leading cause of neonatal EOS. Despite the implementation of IAP against GBS, it remains the leading cause of EOS in term infants. However, coincident with the increased use of intrapartum IAP for GBS, gram-negative enteric bacteria have become the leading cause of EOS in preterm infants. Enteric bacilli causing EOS include E. coli, other Enterobacteriaceae (Klebsiella, Pseudomonas, Hemophilus, and Enterobacter species) and the anaerobe B. fragilis. Less common organisms that can cause serious early-onset disease include Listeria monocytogenes and Citrobacter diversus. Staphylococci and enterococci can be found in EOS but are more commonly causes of nosocomial sepsis and are discussed under that heading in the subsequent text. Fungal species can cause EOS primarily in preterm infants; this is also discussed separately in the subsequent text.
GBS (Streptococcus agalactiae) frequently colonizes the human genital and GI tracts and the upper respiratory tract in young infants. In addition to causing neonatal disease, GBS is a frequent cause of urinary tract infection (UTI), chorioamnionitis, postpartum endometritis, and bacteremia in pregnant women. There is some evidence suggesting that vaginal colonization with a high inoculum of GBS during pregnancy contributes to premature birth.
Microbiology. GBS are facultative diplococci that are easily cultivated in selective laboratory media. GBS are primarily identified by the Lancefield group B carbohydrate antigen and are further subtyped into nine distinct serotypes (types Ia, Ib, II—VIII) by analysis of capsular polysaccharide composition. Most neonatal disease in the United States is currently caused by types Ia, Ib, II, III and type V GBS. Type III GBS are associated with the development of meningitis and are commonly a cause of late-onset GBS disease.
Pathogenesis. Neonatal GBS infection is acquired in utero or during passage through the birth canal. Because not all women are colonized with GBS, documented colonization with GBS is the strongest predictor of GBS EOS. Approximately 20% to 30% of American women are colonized with GBS at any given time. A longitudinal study of GBS colonization in a cohort of primarily young, sexually active women demonstrated that 45% of initially GBSnegative women acquired colonization at some time over a 12-month period. In the absence of IAP, approximately 50% of infants born to mothers colonized with GBS are found to be colonized with this organism at birth. Approximately 1% to 2% of all colonized infants develop invasive GBS disease, with clinical factors such as gestational age and duration of ROM contributing to risk for any individual infant (see subsequent text). Lack of maternally derived protective capsular polysaccharide-specific antibody is associated with the development of invasive GBS disease. Other factors predisposing the newborn to GBS disease are less well understood, but relative deficiencies in complement, neutrophil function and innate immunity may be important.
Table 49.2 Risk Factors for Early-onset Group B Streptococcus (GBS) Sepsis in the Absence of Intrapartum Antibiotic Prophylaxis
Risk factor
Odds ratio (95% CI)
Maternal GBS colonization
204 (100-419)
BW<1,000 g
24.8 (12.2-50.2)
BW<2,500 g
7.37 (4.48-12.1)
Prolonged ROM >18 h
7.28 (4.42-12.0)
Chorioamnionitis
6.42 (2.32-17.8)
Intrapartum fever >37.5°C
4.05 (2.17-7.56)
CI = confidence interval; BW = birth weight; ROM = rupture of membranes. Data from Benitz WE, Gould JB, Druzin ML. Risk factors for early-onset group B streptococcal sepsis: estimation of odds ratios by critical literature review. Pediatrics 1999;103(6):e77.
Clinical risk factors for GBS EOS (see Table 49.2). GBS bacteriuria during pregnancy is associated with heavy colonization of the rectovaginal tract and is considered a significant risk factor for EOS. Black race and maternal age <20 years are associated with higher rates of GBS EOS, although it is not entirely clear whether this reflects only higher rates of GBS colonization in these populations. Multiple gestation is not an independent risk factor for GBS EOS.
Prevention of GBS infection. Multiple trials have demonstrated that the use of intrapartum penicillin or ampicillin significantly reduces the rate of neonatal colonization with GBS and the incidence of early-onset GBS disease. IAP for the prevention of GBS EOS can be administered to pregnant women during labor based on (i) specific risk factors for early-onset GBS infection or on (ii) the results of antepartum screening of pregnant women for GBS colonization. In 2002, the CDC issued guidelines recommending universal screening of pregnant women for GBS by rectovaginal culture at 35 to 37 weeks’ gestation and management of IAP based on screening results. Pregnant women with documented GBS bacteriuria during pregnancy or who previously delivered an infant who developed invasive GBS disease need not be screened as these women should be given IAP regardless of current GBS colonization status. The CDC guidelines for the prevention of earlyonset GBS disease were revised in 2010 to address recent data on neonatal and obstetrical infection management and outcomes (http://www.cdc.gov/groupbstrep/guidelines/guidelines.html).
Highlights of the new guidelines included revised recommendations for the management of neonates at risk for EOS, changes in recommended antibiotic choices for GBS IAP, specific recommendations for mothers who experience preterm labor and premature ROM, expanded laboratory methods for the detection of GBS, including use of alternate culture-based detection methods, and intrapartum nucleic acid amplification testing as an alternative to culture-based detection.
In the 2010 revised guidelines, penicillin and ampicillin remain the recommended antibiotics for GBS IAP. The document addresses the challenges of providing adequate IAP to the roughly 10% of women who reported having penicillin allergy. There is no data directly supporting the efficacy of any antibiotic other than pencillin, ampicillin, or cefazolin for GBS IAP. Erythromycin and clindamycin are frequently given to penicillin-allergic women, but an increasing proportion of GBS isolates (15%—40%) are resistant to these antibiotics. In the penicillin-allergic woman, it is recommended that any GBS isolates identified on screening be tested for antibiotic susceptibility, including specific testing for inducible clindamycin resistance. For the woman with a non—lifethreatening penicillin allergy, cefazolin is the recommended antibiotic for IAP. If a woman has a documented history of anaphylactic penicillin or cephalosporin allergy (including urticaria, angioedema, and/or respiratory distress), clindamycin is recommended if the colonizing isolate is fully susceptible to this antibiotic; otherwise vancomycin is the recommended agent. For the purpose of infant management, however, the 2010 guideline does not consider the administration of clindamycin or vancomycin to constitute fully adequate IAP.
Current status of GBS EOS. The CDC active surveillance data for the United States in 2007 to 2008 demonstrates that the overall incidence of GBS EOS has fallen to 0.28 cases per 1,000 live births (compared with 1.7 cases per 1,000 live births in 1993). There is ongoing racial disparity with the incidence among black infants roughly four times that of white infants. Approximately onequarter of all GBS EOS now occurs among infants born at <37 weeks’ gestation. We evaluated the reasons for persistent GBS EOS despite the use of a screeningbased approach to IAP at the Brigham and Women’s Hospital (BWH). We found that most GBS EOS in term infants now occurs in infants born to women with negative antepartum screens for GBS colonization. Subsequent CDC multistate surveillance studies from 2003 to 2004 found that 61% of GBS disease among term infants occurred in infants born to mothers who screened GBS negative. These “false-negative” screens may be due to improper culture technique or acquisition of GBS between the time of culture and start of labor.
Bacterial culture remains the CDC-recommended standard for detection of maternal GBS colonization. The 2010 revision includes new recommendations for the use of chromogenic GBS detection media and for the use of direct broth detection methods by latex agglutination, probe detection, or nucleic acid amplification testing (NAAT) methods. These approaches may shorten the time to GBS identification. In 2002, the U.S. Food and Drug Administration (FDA) approved the first PCR-based rapid NAAT diagnostic for detection of maternal GBS colonization directly from vaginal/rectal swab specimens. Different kits are now commercially available, and the 2010 guideline endorses the optional use of these NAATs for the management of women whose GBS status is unknown at the time of delivery. Recent data demonstrates that NAATs are more sensitive than antenatal culture in predicting intrapartum GBS status but real-time use is compromised by a 10% incidence of nonresults due to technical issues. Due to the costs and technicalities of providing continuous support for a real-time, PCR-based diagnostic, as well as the inherent time delay in an intrapartum diagnostic, most obstetric services continue to rely on antenatal culture-based screening programs.
Evaluation of infants after maternal GBS IAP. The 2010 revised CDC guidelines include a recommended algorithm for the evaluation of infants born
to mothers exposed to IAP. As in prior versions, the algorithm recommends a full diagnostic evaluation (CBC with white cell differential, LP, CSF and blood cultures, and chest radiograph) and empiric antibiotic therapy for any infant with clinical signs of infection. For asymptomatic infants, a limited evaluation, including CBC/differential and blood culture, and empiric antibiotic therapy are recommended if there were intrapartum signs of maternal chorioamnionitis. Different from previous guidelines, the 2010 version considers only the administration of penicillin, ampicillin, or cefazolin ≥4 hours prior to delivery to constitute adequate IAP. If GBS IAP was indicated but not adequately administered, the revised guideline recommends limited diagnostic evaluation only if other risk factors for EOS are present (gestational age <37 weeks and or ROM ≥18 hours).
Treatment of infants with invasive GBS disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Bacterial and Fungal Infections
Bacterial and Fungal Infections
Karen M. Puopolo
I. BACTERIAL SEPSIS AND MENINGITIS