Attention Deficit, Hyperactivity, and Sleep Disorders
Introduction
Two decades of burgeoning research in pediatric sleep disorders have produced compelling evidence, with broad consequences for public health, that complex relationships among sleep, behavior, and cognition account for serious and common neurobehavioral morbidity in children.1,2 The evidence linking sleep pathology to symptoms of hyperactivity, inattention, and other neurobehavioral deficits is robust and convincing yet replete with contradictions. Seldom is there so much agreement on the scope and significance of a problem with so little consensus on its meaning and mechanism. Here we summarize this work, rationalize it within a multidimensional model, and suggest directions for investigation in the next decade. Several very competent reviews have been published recently that simplify our task considerably and allow us to emphasize a few key concepts.1,3–14
Sleep Disorder and Neurobehavioral Pathology as Comorbid Problems
Parents report sleep problems in 25–50% of their children with attention deficit/hyperactivity disorder (ADHD), according to a widely quoted estimate,15 and depending upon how sleep problems are defined, they may occur in as many as 80%.16 Typical complaints include difficulties falling asleep or returning to sleep, bedtime anxiety or resistance, snoring, restless sleep, enuresis, nightmares, shortened sleep, and daytime sleepiness. A wide range of objective sleep findings, such as reduced sleep efficiency, sleep fragmentation, and elevated apnea–hypopnea index (AHI), have been detected with polysomnography (PSG) and actigraphy. Among children presenting with primary sleep complaints, behavioral symptoms (such as hyperactivity, defiance, and aggression), neuropsychiatric diagnoses (such as ADHD and oppositional defiant disorder (ODD) ), and significant neuropsychological deficits (such as impaired attention and memory, impulsive responding, dull general intelligence, and delayed academic skill development) are common. Parent ratings, sometimes referred to as subjective measurements, are more likely than are objective measures (such as PSG, actigraphy, and multiple sleep latency tests (MSLT) ) to produce substantial correlations with behavioral measures. This is especially true in clinical populations. Community samples of ostensibly normal children assessed by objective means are less likely to generate robust correlations.17
Inconsistencies between subjective and objective measures may reflect differential sensitivities to specific sleep phenomena.18 Parents observe behavior continually over periods of years, whereas the PSG detects behavioral events for a single night, usually in a laboratory setting (and typically one bearing only superficial resemblance to home). An important though seldom appreciated phenomenon affecting parent ratings is the halo effect19 (and its reverse, the infelicitously dubbed devil effect20). These effects occur when high ratings on one desirable (or undesirable) trait are accompanied by unwarranted elevations on other desirable (or undesirable) traits, producing strong but not necessarily true correlations. Abikoff et al.21 showed that teachers observing children with oppositional defiant behaviors, for example, tended to document ADHD symptoms that were not present. Whether this devil effect contributes to the consistently high correlations between daytime behavioral problems and nighttime sleep difficulties in studies based upon parent ratings has not been investigated.
Because ADHD so commonly presents with comorbid psychiatric conditions it has not always been clear whether associated sleep findings are attributable to ADHD or to other psychopathology. Some associations may vanish when children with comorbid disorders such as depression and anxiety are eliminated from subject pools. This possibility was recently examined (2012) by Accardo et al.22 who used a structured interview to diagnose ADHD in 317 children among whom 60 had comorbid anxiety and 62 had comorbid depression. On the Children’s Sleep Habits Questionnaire (CSHQ)23 only the anxious children showed modestly higher total scores relative to the ADHD group without comorbid conditions. Subscale scores, however, revealed sleep onset delay in both comorbid groups, high bedtime resistance and night wakings in the anxious children, and higher sleep duration scores for the depressed group. Comorbidities did not affect ratings on symptoms related to sleep-disordered breathing. Although this study suggested that psychiatric comorbidity is a relevant factor contributing to the frequency of sleep problems in persons with ADHD, it could also be interpreted to indicate that the magnitude of that contribution may be modest and, with respect to symptoms of sleep-disordered breathing (SDB), inconsequential.
Other confounds may account for conflicting findings in the literature. Patterns of sleep and psychiatric morbidity may also depend upon age, sex, obesity and body mass index. Treatment status (i.e., medication in the recent past) introduces the complexity of both direct and rebound effects.24 Difficulties interpreting the literature are further magnified by inconsistencies among experts on important diagnostic thresholds in both sleep and psychiatric symptom criteria. For example, there is at best uneasy agreement on the AHI threshold that should define obstructive sleep apnea.25,26 To some extent, the reconciliation of views depends upon the outcome of research establishing the AHI levels strongly associated with neurobehavioral morbidity. Similarly, the optimal threshold for determining clinical significance of periodic leg movements (PLMs) and associated arousals is not well studied.
Diagnostic inconsistencies in sleep have their counterparts in psychiatric conditions as well. In 1980 the DSM-III – the third edition of the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM)26 – established a radically categorical (and medical) model to define childhood psychopathology. In this system, attributes widely and continuously distributed in the population, such as attention and activity level, are defined as deviant mainly on the basis of quantity or severity rather than quality or nature. Disorders defined in this way have boundaries that are somewhat arbitrary and indistinct, particularly to the extent that the measures of defining traits may be subjective or depend upon judgments of developmental norms.
Sadeh and colleagues12 reviewed reports that used PSGs to compare ADHD children with controls. Multiple confounds were examined including age, sex, rigor of psychiatric diagnosis, recruitment source, medication status, and use of an adaptation night prior to PSG. Studies with manifest recruitment bias were eliminated, but presence of comorbidity was not an exclusion criterion. Twelve samples were analyzed including data from 333 children with ADHD and 231 controls. After sleep time, sleep efficiency, sleep latency, sleep architecture, AHI and respiratory distress indices (RDI), arousals, and PLMs were examined, only PLMs statistically separated ADHD from control children, though the effect size of 0.26 was quite modest. Studies with older subjects and using more rigorous psychiatric diagnostic protocols contributed most to this result. Only seven studies contributed to the PLMs analysis, and a handful of additional subjects negative for PLMs would have moved the results into the statistically non-significant range. In a subset of studies analyzed by Sadeh in which psychiatric comorbidities had been excluded, ADHD children had longer sleep latencies than controls.
Cortese and colleagues8 subsequently conducted a meta-analysis that involved a broader array of variables (such as PSG, actigraphy, MSLT, and subjective measures), and they included several published studies that had been unavailable a few years earlier. They excluded studies that lacked explicit diagnostic criteria and studies with subjects who were medicated or who had comorbid anxiety or depressive disorders. This left 722 ADHD children and 638 controls in 16 reports. The PLMs were not analyzed. Table 15.1 shows 11 significant effects distinguishing ADHD from control children in these analyses, along with the small number of studies (only three to four on average) upon which each conclusion was based (an indication of the slender thread upon which we can base current opinion). The ADHD children displayed longer sleep onset latency when measured by actigraphy but not by PSG. Sleep efficiency, stage shifts per hour, and true sleep time also differed between groups, but sleep onset latency measured by PSG, and percentage of time in stages 1, 2, slow-wave, and rapid eye movement (REM) sleep, did not. The AHI was higher in ADHD subjects and was in accord with subjective parent-reported measures for SDB.
Table 15.1
Positive Sleep Findings in Attention-Deficit/Hyperactivity Disorder
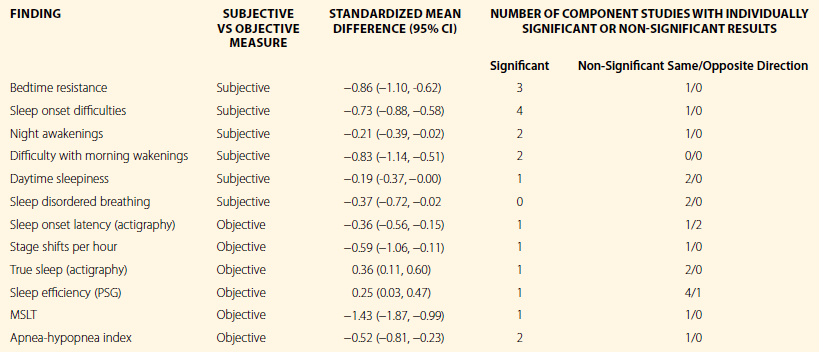
This table, which is based on Cortese S, Faraone SV, Konofal E, Lecendreux M. Sleep in children with attention-deficit/hyperactivity disorder: Meta-analysis of subjective and objective studies. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48:894–908,8 shows positive findings extracted from figure 2 in that paper.
Attention-Deficit/Hyperactivity Disorder: Heterogeneity, Symptoms, and Disorders
Attention deficit disorder with and without hyperactivity (ADD w/ H and ADD w/o H) was introduced in the DSM-III largely on the expectation that the core symptom was inattention and that the ADD w/o H phenotype would prove to be an alternative expression, perhaps more common in girls, of the same presumably familial condition. Numerous studies spanning nearly 30 years have consistently highlighted the distinctiveness of ADHD that is characterized by inattention alone from the combined and hyperactive–impulsive types. The inattentive type tends to be sluggish, anxious, shy, socially withdrawn, unpopular, and poor in sports and academics. Compared to hyperactive children, they have fewer externalizing problems and cause less family stress.27–30 Impressions drawn from descriptive psychopathology have more recently been buttressed by sophisticated imaging methodologies – such as magnetic resonance spectroscopy31 and functional magnetic resonance imaging (fMRI)32 – that have been able to discriminate between central physiological correlates of inattentive and combined types of ADHD.
Sleep characteristics also differ in the two major ADHD types. Chiang et al.33 interviewed youth aged 10–17 in whom they established diagnoses of ADHD-combined in 174, ADHD-inattentive in 130, and ADHD-hyperactive-impulsive in 21. Compared to 257 non-ADHD controls, combined and inattentive ADHD groups were more prone to daytime napping and to sleep disorders generally, but the combined type alone displayed circadian rhythm problems and sleep talking, whereas the inattentive type had more symptoms of hypersomnia. The distinction between ADHD types associated with movement may prove especially relevant to understanding the connection between ADHD and disorders such as restless legs syndrome for which the urge to move is a defining feature.
The problem of heterogeneity in ADHD does not end with the types specified in the DSM.34 Within the combined ADHD phenotype multiple genetic and non-genetic variants may well be discovered.35 There is no need for a single grand hypothesis that explains ADHD or each of its relationships to diverse sleep disorders through a single mechanism. There is room for multiple hypotheses to guide different aspects of future investigation. Comorbidities with sleep disorders may be an important pathway for discriminating homogeneous ADHD phenotypes that would improve the specificity and biological relevance of psychiatry’s classification system.
Nature of Interactions between Sleep and Neurobehavioral Pathology
Figure 15-1 diagrams a few of the multiple pathways proposed as explanations for the comorbidity of sleep disorders and neurobehavioral pathology. The potential for these mechanisms to interact can be easily appreciated from a glance at this picture, though our understanding of these complex interactions is quite limited.
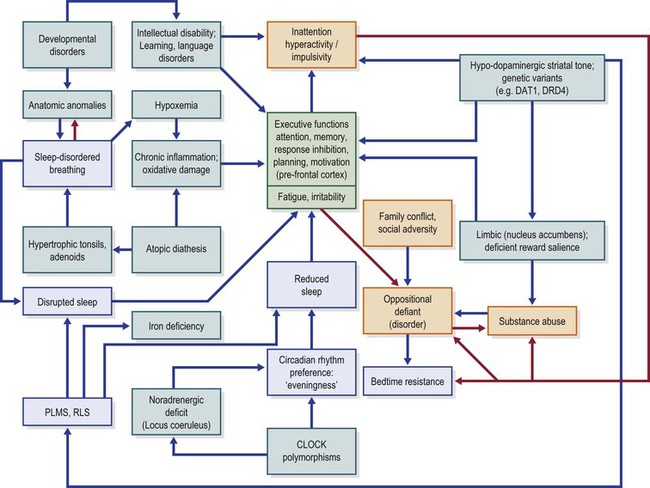
Figure 15-1 Model of Hypothetical Causal Pathways Linking Sleep Disorders with Inattention and Hyperactivity.
PLMS, periodic limb movements in sleep; RLS, restless legs syndrome; DAT1, dopamine transporter 1; DRD4, dopamine receptor D4; CLOCK, Circadian Locomotor Output Cycles Kaput gene. Blue boxes, sleep disorders; yellow boxes, behavioral pathology; green boxes, biological cognitive dysfunction and biological factors. Blue arrows show possible functional or causal sequences; red arrows show positive feedback loops. Deeper green colored boxes (near the center of the diagram) show executive functions as the common pathway of several causal chains, with fatigue and irritability as closely allied subjective experiences.
ADHD and Stimulants as a Cause of Sleep Dysfunction
It is not surprising, therefore, that difficulties initiating or maintaining sleep represent the most common sleep complaints related to ADHD. These complaints often fall diagnostically into the International Classification of Sleep Disorders-Second Edition (ICSD-2)36 grouping of behavioral insomnias of childhood (BIC), especially the limit-setting type which develops when the caretaker rewards avoidance behaviors by allowing the child to delay bedtime.
The dynamics of BICs are well understood in terms of behavioral theory, the validity of which is supported by the effectiveness of behavioral interventions such as extinction (which usually means ignoring protests or other entreaties for attention), graduated extinction, positive bedtime routines with fading in of a target bedtime, scheduled awakenings, and parent education. A committee of the American Academy of Sleep Medicine (AASM) reviewed the literature and adapted the most effective techniques in a practice parameter to guide treatment interventions for preschool children, though nothing comparable exists specifically for ADHD.37,38 Surprisingly, no trials have been published with appropriate controls proving the efficacy of a systematic behavioral approach to BIC in children with ADHD. Preliminary, unpublished results for a manualized 5-week intervention suggest that children with ADHD respond as well as others.16 Another trial that compared two versions of an intervention geared to ADHD did not include an untreated control group.39
Although sleep initiation problems in most children with ADHD have been understood and treated largely on the basis of learned behavior, family conflict, and medication effects, the extent to which endogenous cycles contribute to bedtime resistance in some children with ADHD may deserve closer attention than it has previously received. Delayed sleep phase disorder typically becomes most severe in or after adolescence, but similar tendencies also can be identified in younger children.40,41 Interestingly, in a sample of 5- to 14-year-old ADHD children with common sleep disorders who had been recruited for a pilot trial of a simple behavioral intervention, 56% were diagnosed with delayed sleep phase while only 28% were diagnosed with limit-setting disorders.39
Medications for ADHD have varying impacts on bedtime compliance. Atomoxetine, clonidine, and guanfacine all promote sleep, and the latter two are sometimes administered primarily for that purpose. Stimulants may affect sleep directly by promotion of alertness and reduction of sleepiness – even after the major therapeutic effects have disappeared – by aggravation of symptoms during a period of rebound hyperactivity that extends into the child’s bedtime.42 Or, somewhat paradoxically, they may facilitate sleep through a direct therapeutic effect on the hyperactivity. For some children the impact of stimulants is tantamount to a chemically induced phase delay. When medication underlies a serious sleep problem, simple behavioral interventions such as educating parents and improving sleep hygiene, though potentially of benefit, may be inadequate without changes in the medication regimen. Some evidence exists that the longer-acting stimulants are less likely to trigger problems in sleep initiation.24
An Intrinsic Sleep Disorder as the Hypothetical Etiology of Inattention, Hyperactivity, and Other Neurocognitive Impairment: Sleep-Disordered Breathing
Even brief experimental restrictions of sleep can affect attention and cognition in healthy children. Thus, a single night’s experimental reduction in sleep produced inattentive behaviors43 and impairment in higher cognitive functions such as verbal fluency and flexibility and acquisition of abstract concepts.44 Similarly, during a week of restricted sleep, normal children displayed decrements in teacher-rated academic and attention scores, though without changes in hyperactivity or oppositional behavior.45 Gruber et al.46 reported that parent ratings of alertness, and teacher ratings of emotional regulation and restless-impulsive behavior, improved among children aged 7–11 who had extended their sleep time by an average 27.36 minutes during an experimental trial lasting a week. In contrast, children who restricted sleep by an average of 54.04 minutes deteriorated with respect to these measures.
Sleepiness due to either sleep disruption or reduced sleep duration is a common problem among children with ADHD symptoms. A survey of parents of more than 800 children at general pediatric clinics, for example, found a substantial association between sleepiness and behaviors that characterize ADHD,2 while MSLT has objectively confirmed this observation in three studies employing this measure.47–49 Actigraphic studies of sleep onset latency and sleep duration have produced mixed findings50 but at least two reports, not confounded by medication effects, have shown sleep latency to be greater,51 and sleep duration to be shorter,52 in ADHD children than in controls.
Behavior is largely the product and manifestation of high-level cognitive functions, collectively referred to as executive functions, that influence self-control, reasoning, and judgment. One formulation of these functions defines six major domains that include inhibition, set shifting, self-regulation of affect and arousal, working memory, analysis/synthesis, and contextual memory.53 In some measure, impaired executive function is present in all persons with ADHD, at least to the extent that the defining features of ADHD can be said to be executive functions per se (namely the capacities to control attention and to regulate behavior, including modulation of activity and impulse). A more granular dissection of cognition usually will identify additional features of executive dysfunction among children with the ADHD diagnosis, but at its core it can be said that ADHD is executive dysfunction.
Impaired executive function can result from disordered sleep or daytime sleepiness. In a model posited by Beebe and Gozal,53 the disrupted sleep and intermittent hypoxia and hypercarbia resulting from obstructive sleep apnea impair restorative sleep and cellular homeostasis. These effects, in turn, cause prefrontal cortical dysfunction manifested in executive dysfunction. A neurobehavioral phenotype emerges with difficulty manipulating information, poor planning and execution, disorganization, poor judgment, rigid thinking, poorly maintained attention and motivation, emotional lability, and overactivity/impulsivity. This description is generally consistent with a diagnosis of ADHD, and many cases of ADHD would neatly fit such a description.
The prefrontal cortex, the brain region thought to be most responsible for executive functions, is particularly vulnerable to sleep deprivation. Thus, sleep-deprived adults have paradoxical prefrontal activation demonstrable on fMRI during a verbal learning task.54 In contrast, subjects with OSA have shown reduced prefrontal activation in a brief visual delayed matching-to-sample task.55 Even where neuropsychological deficits are mild, adults with OSA may show widespread functional changes in cerebral cortex, including in prefrontal regions.56 Functional and anatomic imaging of children and adults with ADHD has shown prefrontal cortical abnormalities consistent with the neuropsychological models of executive function.57–60
Gozal61 has extended the executive function model of sleep and ADHD to emphasize the role of inflammation and oxidative stress as potential mechanisms for cellular changes underlying neurocognitive deficits in children with OSA. This perspective is supported by experimental rodent studies showing that intermittent hypoxia leads to cellular damage, including changes in gene expression,62,63 and to behavioral outcomes similar to those observed in OSA. Furthermore, in children with OSA, and especially those with snoring and with neurocognitive deficits, levels of C-reactive protein, found in high-sensitivity testing, are elevated.64 In addition, in non-obese children with OSA, levels of soluble CD40 ligand – an indicator of endothelial inflammation – are also high.65 Insulin-like growth factor 1 (IGF-1), a neuroprotective hormone, is also elevated in children with OSA, more so in children with less neurocognitive dysfunction who are presumably benefitting from greater neuroprotection provided by higher hormone levels.66 Among children with ADHD but without reported sleep problems, a role for oxidative stress and cellular immunity has been suggested as well. Thus, 35 children and adolescents with ADHD were found to have high nitric oxide synthetase, xanthine oxidase, and adenosine deaminase activities but low glutathione S-transferase and paraoxonase-1 activity.67 The importance of inflammation may be related to the apparent excess of atopic disorders and food sensitivities among children with ADHD.68–73 Although fad diets – such as the Feingold diet74,75 – have rarely survived rigorous clinical trials, elimination diets have consistently identified children with behavioral sensitivities to specific foods or ingredients that can be demonstrated on repeat food challenges.76,77 Practicability, perhaps more than noteworthy weaknesses in the evidence, has prevented these approaches from entering mainstream practice or attracting a large body of research.78
There is little doubt that the severest forms of pediatric obstructive sleep apnea can cause a characteristic daytime syndrome comprising irritability, languor, and cognitive dulling with prominently impaired attention and memory. Osler79 vividly described the impact of adenoid hypertrophy in his classic text:
Many publications suggest that PSG measures such as the AHI, usually considered to be valid indices of the presence and severity of nocturnal respiratory disturbance, may fail to detect labored nocturnal respiration that is relevant for neurobehavioral pathology. A German study, for example, has challenged the assumption that primary snoring – which by definition is not associated with significant apnea, hypopnea, or associated arousals – is benign.80 Of 92 habitual snorers studied (from a community population of 1114 school-age children) 69 had primary snoring and the remaining 23 had either upper airway resistance syndrome (UARS) or OSA. All three of these objectively diagnosed forms of SDB were associated with significant neurobehavioral morbidity – including both hyperactivity and inattention – to about the same extent. Primary snoring was an important risk factor for hyperactivity (OR = 2.8), inattentive behavior (OR = 4.4), daytime sleepiness (OR = 10.7), and diminished performance in mathematics, science, and spelling.
Like Osler’s case, most prepubertal children with significant SDB have enlarged adenoids and tonsils for which adenotonsillectomy is often recommended. Several groups have systematically examined the consequences of adenotonsillectomy in children with putative SDB. Huang et al.80A offered children with ADHD and mild OSA, defined by an AHI between one and five events/hour, the choice of adenotonsillectomy, conventional stimulant treatment for ADHD, or no treatment. The group choosing surgery had the best outcome on the ADHD rating scale and on a continuous performance test, leading the authors to conclude that mild SDB warranted surgical treatment. Chervin and colleagues81 studied children selected to undergo adenotonsillectomy on surgical grounds before formal psychiatric and sleep assessment had been performed. Comprehensive diagnostics including PSG with esophageal pressure monitoring, MSLT, neuropsychological testing, and structured psychiatric interviews, took place immediately before surgery and about 1 year later. In most instances clinician-diagnosed SDB had been an indication for surgery, though only about half the children were subsequently diagnosed with OSA (even when only requiring an obstructive apnea index of 1). Both neurobehavioral measures and DSM-IV diagnoses improved substantially after surgery. At baseline, 37% of 79 subjects compared to 11% of 27 controls were assigned diagnoses of attention or disruptive behavior disorders, whereas a year later only 23% of post-surgical cases still met criteria for at least one of these diagnoses.82 Figure 15-2 summarizes diagnostic changes before and after surgery. ODD, a disorder that is characterized by traits of irritability and proneness to protests and tantrums, was particularly amenable to improvement through surgery. These findings were replicated in a second cohort.83
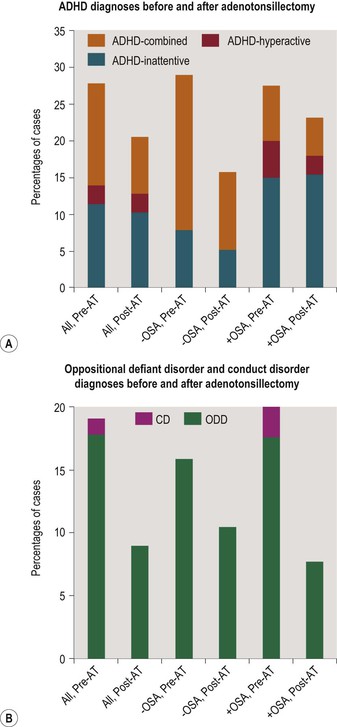
Figure 15-2 Figures show changes in DSM-IV diagnostic rates of disorders of attention and disruptive behavior before and about 1 year after adenotonsillectomy in 79 children aged 5 to 12. The bars show stacked rates for ADHD types (2a), and for disruptive behavior disorders (2b), both in cases without polysomnographic evidence of OSA, and in those with such evidence (obstructive apnea index of 1 or more). ADHD, attention-deficit/hyperactivity disorder; ADHD-hyperactive, predominantly hyperactive/impulsive type; ADHD-inattentive, predominantly inattentive type; CD, conduct disorder; ODD, oppositional defiant disorder; OSA, obstructive sleep apnea; −OSA, no OSA present on polysomnography; +OSA, OSA present. AT, adenotonsillectomy. ‘All’ refers to 79 subjects undergoing AT, whereas the −OSA group comprised 38 subjects and the +OSA group 40.
* p < .05 (McNemar’s test). Data for this figure have been adapted from Dillon JE, Blunden S, Ruzicka DL, Guire KE, Champine D, Weatherly RA, et al. DSM-IV diagnoses and obstructive sleep apnea in children before and 1 year after adenotonsillectomy. Journal of the American Academy of Child & Adolescent Psychiatry 2007;46:1425–36.82
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


