Approach to the Cyanotic Infant
Robin H. Steinhorn
ASSESSMENT
Cyanosis, or bluish discoloration of the skin, is derived from the Greek word kuaneos, meaning dark blue. Cyanosis is caused by the presence of deoxygenated hemoglobin in the blood vessels that is most visible on the surface of the skin and mucosa. In general, cyanosis occurs because (1) the binding of oxygen to hemoglobin is abnormal so that blood does not carry much oxygen despite having a normal partial pressure of oxygen, or PO2 (eg, methemoglobin or carboxyhemoglobin); (2) the perfusion of the skin is poor, such that the venous and capillary blood are very deoxygenated even though the arterial blood may be well oxygenated (eg, cold environment or circulatory shock); or (3) the arterial, and therefore the capillary and venous blood, is poorly oxygenated (eg, a right-to-left shunt with congenital cardiac disease, parenchymal pulmonary disease, or hypoventilation). Cyanosis tends to become apparent when there is about 3 to 5 g/dl of deoxygenated hemoglobin, but detection varies widely depending on lighting, observer differences, and pigmentation of the skin, among other factors. The oxygen binding capacity of the fetal hemoglobin in the newborn also alters the degree of desaturation at a given PaO2. For example, at a PaO2 45, the saturation of adult hemoglobin would fall below 80%, typically creating a cyanotic appearance but fetal hemoglobin saturation would remain in the mid 80s, which may not be associated with overt cyanosis (see eFig. 49.1  ). There is urgency to determine the cause of the cyanosis because of the high risk of tissue injury or death posed by poor oxygenation and in order to guide important interventions to improve tissue oxygenation. Although a specific diagnosis may not necessarily be determined at the bedside without special studies, the underlying nature of the disturbance usually can be derived with common clinical tools and the physical examination.
). There is urgency to determine the cause of the cyanosis because of the high risk of tissue injury or death posed by poor oxygenation and in order to guide important interventions to improve tissue oxygenation. Although a specific diagnosis may not necessarily be determined at the bedside without special studies, the underlying nature of the disturbance usually can be derived with common clinical tools and the physical examination.
Arterial O2 saturation using pulse oximetry should immediately be measured in any cyanotic newborn infant. It is particularly important to measure blood saturation of tissue that is likely perfused from the aorta proximal to the ductus arteriosus—generally, the right hand or, if possible, an ear lobe—and from a lower extremity. Although there is some imprecision in oximeters, especially if perfusion is poor (see Chapters 103 and 106), this approach will help establish if the hypoxemia is a valid finding. Furthermore, right-to-left shunting across the ductus arteriosus may be detected when the upper and lower body saturations differ consistently by more than 3% to 5%. If there is indeed hypoxemia, measurement of arterial blood gas tensions and pH is also important to help determine whether there is an acidemia of respiratory (from hypoventilation or fatigue) or metabolic (from tissue hypoxia) origin.
At the same time, a rapid assessment of the circulatory and respiratory systems can establish a few important issues. By evaluating central and peripheral pulses, capillary refill, and extremity warmth (see Chapter 103), it should be apparent whether poor perfusion with peripheral vasoconstriction is contributing to the cyanotic appearance. The clinician should simultaneously assess the breathing pattern to determine if the child has signs of respiratory distress or quiet and unlabored respiration. Pulmonary edema, alveolar consolidation, or collapse sufficient to cause cyanosis is invariably accompanied by an increase in inspiratory work, including tachypnea, retractions, grunting, accessory muscle use, or nasal flaring (see Chapter 102). In contrast, the child with normal pulmonary mechanical function would be expected to breathe somewhat faster and deeper than normal in the presence of hypoxemia but would not have signs of markedly increased work. Finally, if an infant has slow and shallow respirations and appears to have depressed responses, this might be caused by respiratory depression, in which case the hypoxemia is caused by hypoventilation.
Table 49-1. Pathogenesis of Cyanosis in the Newborn Infant
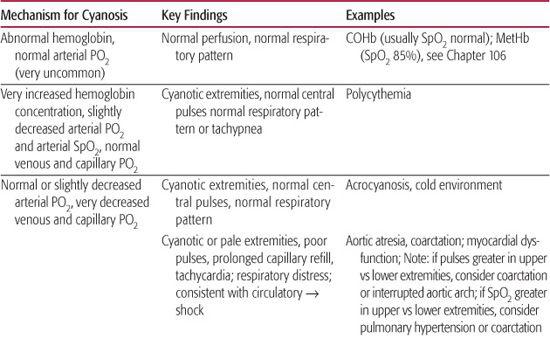
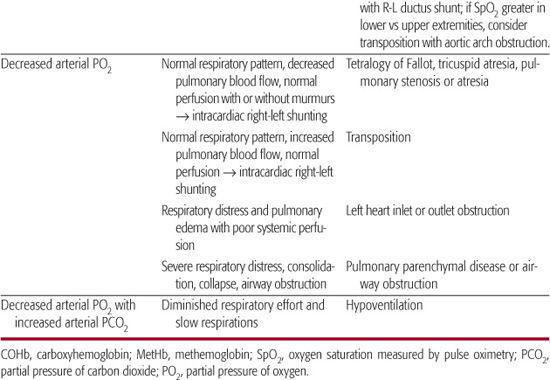
Based on the initial oximetry and blood gas data and the physical examination, a few preliminary conclusions might be derived (Table 49-1). 
THE DIFFERENTIAL DIAGNOSIS
Utilizing an ABC (Airway, Breathing, Circulation) algorithm of evaluation permits a rapid and systematic consideration of the most common causes of cyanosis in the newborn period (Table 49-2). It is important to recognize that cyanosis is more difficult to recognize at lower hemoglobin values due to the altered concentration of deoxygenated hemoglobin as shown in eTable 49.1  .
.
 AIRWAY: UPPER AND LOWER AIRWAY DISEASE
AIRWAY: UPPER AND LOWER AIRWAY DISEASE
Abnormalities of the airway will generally present shortly after birth.4,5 Choanal atresia occurs in about 1 in 5000 infants, with unilateral disease being more common. Choanal atresia should be suspected when an infant’s distress is more obvious in a quiet state and improves during crying. It can be confirmed by the inability to pass a suction catheter through the nares into oropharynx, as well as by computer tomography scanning. Placement of an oral airway should provide immediate improvement. Other associated anomalies are very common; in particular, CHARGE sequence (coloboma, heart disease, atresia of choana, retarded growth and development, genitourinary anomalies, ear/hearing anomalies) should be considered.
Micrognathia, retrognathia, or the Pierre Robin sequence generally presents early in life and will be obvious on physical examination. The airway obstruction from the posterior tongue is more pronounced in supine position.
Laryngomalacia is a congenital abnormality of the larynx and is the most common cause of inspiratory stridor in infants. While it may be noted immediately after birth, it commonly presents at several weeks of age. Airway symptoms typically worsen with crying, feeding, and respiratory infections. Subglottic stenosis may occur as a congenital malformation or be acquired after prior airway manipulation. Infants present with stridor, respiratory distress, or obstructive apnea.
Vocal cord paralysis may occur in association with birth or surgical trauma and is another common cause of stridor in the newborn. It is typically unilateral, causing a hoarse cry and minimal respiratory symptoms. In contrast, bilateral vocal cord paralysis can cause severe respiratory distress, and a tracheostomy may be required. In these cases, central nervous system anomalies such as the Arnold-Chiari malformation should be considered.
Table 49-2. Common Causes of Cyanosis
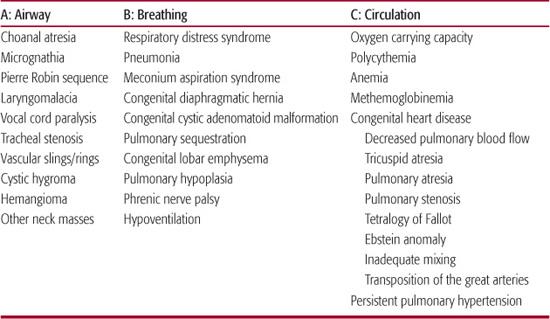
Other conditions may cause intrinsic or extrinsic compression of the trachea. Tracheal stenosis is characterized by inspiratory and expiratory stridor, respiratory distress, wheezing, and persistent cough.  Abnormal development of the great vessels (eg, vascular ring) can compress or deviate the trachea, causing airway obstruction.
Abnormal development of the great vessels (eg, vascular ring) can compress or deviate the trachea, causing airway obstruction.  The trachea can also be compressed by masses such as teratomas, cystic hygromas, or hemangiomas.
The trachea can also be compressed by masses such as teratomas, cystic hygromas, or hemangiomas.
 BREATHING: LUNG DISEASE
BREATHING: LUNG DISEASE
Respiratory distress syndrome (RDS; see also Chapter 54), also known as hyaline membrane disease, occurs almost exclusively in premature infants. 
Neonatal pneumonia (see Chapter 50) is most commonly acquired at the time of birth and usually causes diffuse rather than lobar infiltrates. The initial radiograph is frequently indistinguishable from the ground-glass appearance of RDS, although pleural effusions are more characteristic of pneumonia. Bacterial pneumonia is most common, and frequent pathogens include group B β-hemolytic streptococci (GBS) and gram-negative enteric bacilli (Escherichia coli, Klebsiella, Enterobacter). 
Approximately 13% of all live births are associated with meconium stained fluid, although only 5% of these infants develop meconium aspiration syndrome. Meconium aspiration can injure the lung through multiple mechanisms, including mechanical obstruction of the airways, chemical pneumonitis, inactivation of surfactant, and vasoconstriction of pulmonary vessels, all of which prevent adequate ventilation and oxygenation in the immediate postnatal period. 
Congenital lung abnormalities are rare but important causes of respiratory distress in the newborn.5 Congenital diaphragmatic hernia is a relatively common birth defect. Due to its frequent association with significant pulmonary hypoplasia and pulmonary hypertension, infants with congenital diaphragmatic hernia typically present shortly after birth with respiratory distress. Congenital cystic adenomatoid malformations (CCAM) are extremely rare lung abnormalities composed of cystic lung tissue with communication to the bronchial tree.  Congenital lobar emphysema (CLE) is an over-inflated, hyperplastic area of the lung surrounded by otherwise normal lung tissue. These are most common in the upper lobes. Symptoms are progressive but are occasionally evident at birth.
Congenital lobar emphysema (CLE) is an over-inflated, hyperplastic area of the lung surrounded by otherwise normal lung tissue. These are most common in the upper lobes. Symptoms are progressive but are occasionally evident at birth.
Respiratory failure and cyanosis may occur secondary to neurological dysfunction. For instance, birth injury associated with neurological depression or hypoxic-ischemic encephalopathy is commonly associated with hypoventilation. Hypoglycemia may cause central nervous system depression and secondary respiratory distress; this is most commonly seen in small-for-gestational-age infants, large-for-gestational-age infants, infants of diabetic mothers, infants with birth asphyxia, or in rare cases due to primary hyperinsulinism (eg, nesidioblastosis or Beck-with-Wiedemann syndrome). 
 CIRCULATION: CARDIAC AND CIRCULATORY CAUSES
CIRCULATION: CARDIAC AND CIRCULATORY CAUSES
Polycythemia can cause pulmonary hypertension due to increased viscosity of the blood interfering with pulmonary perfusion. This may be seen in infants of diabetic mothers, in the presence of delayed clamping of the umbilical cord or chronic fetal hypoxia (eg, placental insufficiency, preeclampsia), in recipient twins of twin-to-twin transfusion syndrome, and in conditions such as trisomy 21.
Abnormalities of the hemoglobin molecule itself may interfere with the normal chemical combination of hemoglobin with oxygen, but these are very rare in neonates. The most common cause is methemoglobinemia, which results from the oxidation of hemoglobin molecules from the normal ferrous to ferric state. Infants are more susceptible to methemoglobinemia because fetal hemoglobin is more easily oxidized than is adult hemoglobin and because levels of methemoglobin reductase are relatively low in infants. Methemoglobinemia may result from exposure to oxidants (eg, nitrites, sulfonamides, prilocaine, metoclopropamide) or, rarely, from congenital deficiency of methemoglobin reductase. The characteristic clinical scenario is a blue-gray-appearing infant without respiratory distress who has decreased oxygen saturation but normal arterial oxygen tension.
Severe cyanosis is a prominent feature of congenital heart disease associated with separate circulations and poor mixing (e.g. aortopulmonary transposition) or complete mixing but diminished pulmonary blood flow.7 In the case of complete mixing (eg, tricuspid or aortic atresia), arterial oxygen saturation is a function of the relative amounts of pulmonary and systemic blood flow and the relative oxygenation in the pulmonary and systemic venous return. Hence, hypoxemia can result from markedly diminished pulmonary blood flow; however, hypoxemia can also result from exuberant pulmonary blood flow with pulmonary edema and poor systemic blood flow, which reduces the oxygenation of systemic venous return. Thus, cardiac disease associated with complete mixing may be associated with a variable degree of cyanosis. In any of these conditions in which pulmonary blood flow is dependent on blood directed to the lungs through a patent ductus arteriosus, cyanosis can worsen at the time of ductus closure and tends to improve rapidly after the ductus is reopened following initiation of prostaglandin E1 (PGE1). 
Persistent pulmonary hypertension of newborn (PPHN) describes the failure of the normal circulatory transition that occurs after birth3 and is characterized by marked pulmonary hypertension that causes hypoxemia and right-to-left extrapulmonary shunting of blood through fetal channels (foramen ovale and ductus arteriosus). The combination of inadequate pulmonary perfusion and extrapulmonary shunting leads to severe, refractory hypoxemia. PPHN often complicates parenchymal lung diseases can also occur as an idiopathic condition.
INITIAL DIAGNOSTIC EVALUATION
A detailed physical examination should be performed when the infant is appropriately warmed and quieted. The growth characteristics should be noted on physical examination, because infants who are small or large for gestational age are more prone to polycythemia. The initial focus is on determining the degree of respiratory distress and systemic perfusion. Because neurological conditions are potential causes of cyanosis due to hypoventilation and may be associated with slow or irregular respirations, it is also important to evaluate the infant’s tone and activity and to assess for periodic breathing and/or apneic spells.
The cardiac examination is described in Chapter 44. Although auscultation of heart murmurs is often not helpful, attention to the second heart sound, which will be loud and single (or narrowly split) in pulmonary hypertension, as well as transposition and pulmonary atresia, can be helpful.
A chest radiograph is an integral part of the initial assessment of the cyanotic newborn. The locations of stomach, liver, and heart should be determined to rule out dextrocardia and situs inversus. Examining the lung fields may reveal parenchymal lung disease or lung abnormalities such as cystic adenomatoid malformation. Elevation of either hemidiaphragm by more than 2 intercostal spaces relative to the opposite side suggests diaphragmatic paralysis due to phrenic nerve injury. Hyperinflated lung fields are seen occasionally in lobar emphysema or cystic lesions of lungs. Decreased pulmonary vascular markings are characteristic of pulmonary stenosis or pulmonary atresia with inadequate ductal shunting and may be seen in infants with idiopathic persistent pulmonary hypertension of the newborn. The size and shape of the heart may yield some clues to the diagnosis, for example, the cardiomegaly characteristic of Ebstein anomaly.
An electrocardiogram can occasionally be useful to determine the absence of ventricular forces, which suggests a cardiac anomaly (eg, an infant with left axis deviation and reduced right ventricular forces, which suggests tricuspid atresia). Normal newborns have a predominance of right-sided forces, and moderate right ventricular hypertrophy is a common finding with many types of respiratory and cardiac disease. Therefore, the electrocardiogram can also be completely normal even in infants with serious disease such as transposition.
Some advocate for the hyperoxia test as a clinical tool to differentiate between pulmonary and cardiac disease in cyanotic infants. The test is based on the principle that in the absence of fixed cardiac shunts, 100% oxygen will increase alveolar PO2, leading to an increase in pulmonary venous and systemic arterial PO2. In cyanotic congenital heart disease (eg, decreased pulmonary blood flow or transposition of the great arteries), little or no rise in PaO2 would be expected after breathing 100% oxygen. However, there can be limited increase in PaO2 with parenchymal lung disease or persistent pulmonary hypertension. Given the current wide availability of echocardiography, the hyperoxia test should rarely (if ever) be necessary and should be considered only after discussion with a cardiologist.
INITIAL TREATMENT
Severe cyanosis requires urgent supportive therapy while a diagnosis is established.  An airway and assisted ventilation should be considered for infants with respiratory distress but should be deferred for the infant breathing comfortably. If the infant is less than 10 days old and the umbilical stump is still attached, umbilical venous and arterial lines can be rapidly placed by experienced practitioners for rapid central access. Hypocalcemia is often associated with cardiac disease and critical illness and should be corrected on the basis of the ionized calcium.
An airway and assisted ventilation should be considered for infants with respiratory distress but should be deferred for the infant breathing comfortably. If the infant is less than 10 days old and the umbilical stump is still attached, umbilical venous and arterial lines can be rapidly placed by experienced practitioners for rapid central access. Hypocalcemia is often associated with cardiac disease and critical illness and should be corrected on the basis of the ionized calcium.
Oxygen should be provided once cyanosis is documented. The Neonatal Resuscitation Program guidelines recommend using 100% oxygen for the infant requiring acute resuscitative measures, although the increasing concerns about the potential risks associated with this therapy are worth noting8 (see Chapter 42). Therefore, except in the scenario of acute resuscitation, the use of 100% oxygen should generally be avoided at the outset. Initiating oxygen therapy with 40% to 60% oxygen will allow the caregiver to provide support, assess for improvement, and seek advice from a cardiologist. This point is particularly important if an infant has only a minimal response to oxygen, which may indicate potential cardiac disease and need for prostaglandin E1. Under these circumstances, it is important to remember that oxygen may promote ductal closure. This may not be a major concern for lesions that limit pulmonary blood flow, as the pulmonary venous PO2 would not be expected to rise much. However, admixture lesions such as hypoplastic left heart syndrome may present with moderate cyanosis. These conditions are dependent on a patent ductus to maintain systemic blood flow. Oxygen not only may promote ductal closure but also may increase pulmonary and decrease systemic blood flow (see Chapter 484). 
Prostaglandin E1 is clinically effective for infants dependent on ductal patency to maintain pulmonary blood flow or sufficient mixing. It is given intravenously by constant infusion, and the initial dose is typically 0.05 mcg/kg/min. Apnea is a common side effect after initiation of prostaglandin E1, and some recommend intubation if the infant will require transport shortly after beginning the infusion. Other common side effects include flushing and diarrhea. There are no absolute contraindications to beginning prostaglandin, although it may worsen the pulmonary edema associated with obstructed total anomalous pulmonary venous return (eFig. 49.1  ).
).
REFERENCES
See references on DVD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


