Introduction
Headache is a common symptom among pregnant women. Differentiating between benign, often chronic, headache syndromes and acute conditions signifying more serious pathophysiology is crucial. A careful history and physical exam can often aid the clinician in distinguishing between chronic and acute headache syndromes in pregnant women. Likewise, diagnostic imaging and the appropriate use of medical therapy need to be tailored to pregnancy, to avoid known teratogenic, uterotonic or otherwise potentially harmful agents.
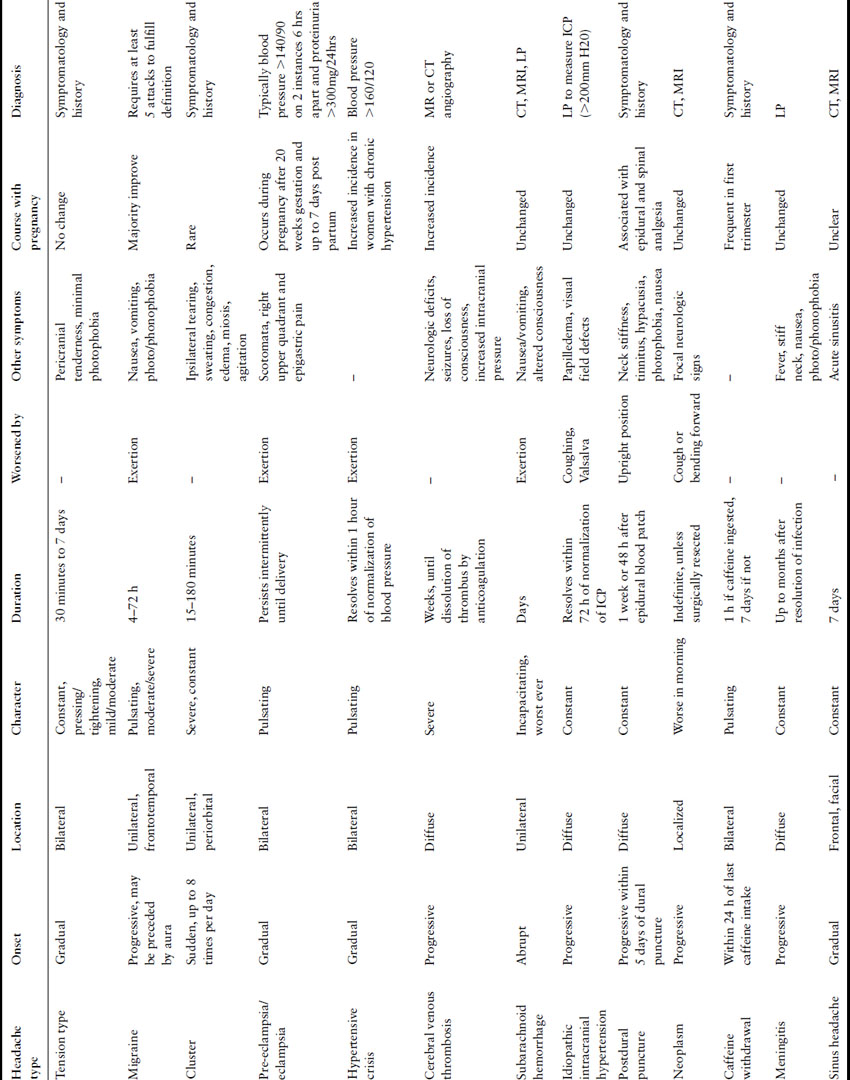
In 2004, the International Headache Society (IHS) presented an updated classification system [1], which categorizes headaches as primary (tension and migraine) or secondary (tumor, trauma) (Box 35.1) and describes the typical signs and symptoms associated with each type (Table 35.1).
Table 35.1 Characteristics of headaches in pregnancy
Box 35.1 IHS classification (2004)
Primary headaches
- Migraine with and without aura
- Tension-type
- Cluster
- Other
- Stabbing
- Cough
- Orgasmic
- Exertional
- Stabbing
Secondary headaches
- Trauma
- Vascular disorders
- Subarachnoid hemorrhage
- Cerebral venous thrombosis
- Vasculitis
- Ischemic stroke
- Vascular malformation
- Arterial dissection
- Subarachnoid hemorrhage
- Intracranial disorders
- Idiopathic intracranial hypertension
- Postdural puncture
- Neoplasm
- Idiopathic intracranial hypertension
- Substance abuse or withdrawal
- Alcohol
- Caffeine
- Cocaine
- Medication overuse
- Alcohol
- Infections
- Meningitis
- Disorders of homeostasis
- Pre-eclampsia
- Hypoglycemia
- Pre-eclampsia
- Disorders of cranial structures
- Sinusitis
- TMJ
- Sinusitis
- Psychiatric disorders
- Somatization
- Psychosis
- Somatization
- Neuralgias
- Trigeminal neuralgia
Chronic headache syndromes, classified as primary headaches by IHS, include tension, migraine, and cluster headaches. Melhado et al. interviewed a prospective cohort of 1101 pregnant women at three points during their pregnancies [2]; 93% reported a history of headache prior to pregnancy. Using the IHS headache classification system, 82.4% had a history of migraine or probable migraine with or without aura and 11.3% had tension-type headaches; 55% of women reported improvement in the first trimester, 60% in the second trimester, and 65% during the third trimester. Of the 7% of women who experienced a new or different headache during the pregnancy, 34.2% were classified as migraine, 2.6% as tension type, and 32.9% as headache secondary to hypertension. The remainder were characterized as secondary (e.g. sinusitis, neck or facial pain, withdrawal from caffeine or medication).
Migraine headache
Severe migraines affect approximately 17% of American women [3]. They are usually characterized as unilateral and pulsating and may be associated with photophobia, phonophobia, nausea, and exacerbation by physical activity [1]. Migraines are associated with fluctuations in estrogen levels and may actually improve during pregnancy when estrogen levels are higher and constant [4,5]. For this reason, migraines tend to recur during the postpartum period when estrogen levels fall, particularly in women with a history of menstrual-associated migraines. Of note, migraines are less frequent in breastfeeding women [6].
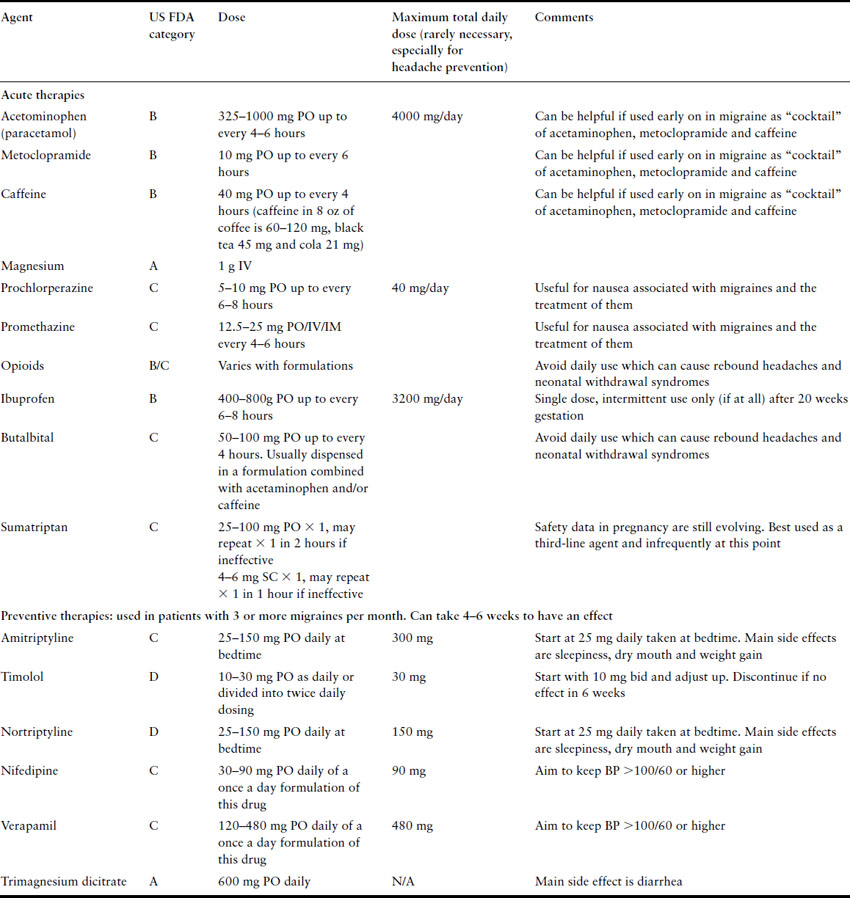
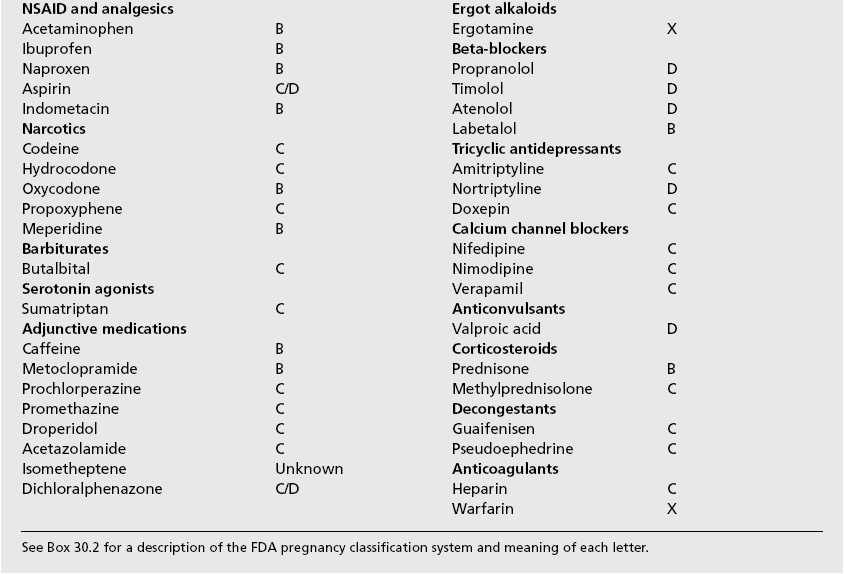
When migraines recur during pregnancy, treatment must consider the effect on the developing fetus. Box 35.2 reviews the components of many combination formulation medications used to treat headaches, and Box 35.3 provides the US Food and Drug Administration’s pregnancy safety category for many of the agents used to treat headaches. Table 35.2 lists many of the agents that may be used to treat or prevent migraines in pregnancy that are reviewed in the following paragraphs.
Table 35.2 Treatment options for migraines in pregnancy
Box 35.2 Components of common headache medications
| Acetaminophen, aspirin, caffeine |
| Acetaminophen, butalbital, caffeine |
| Aspirin, butalbital, caffeine |
| Acetaminophen, hydrocodone |
| Acetaminophen, propoxyphene |
| Acetominophen, isometheptene, dichloralphenazone |
Box 35.3 US FDA classification of medications used to treat headaches and associated conditions
Acetominophen is a first-line agent as it is considered safe for the mother and fetus. A dose of 1000 mg has been shown to be effective in reducing pain, photophobia, phonophobia, and is also effective in improving daily functioning [7]. Other medications may be given in combination with acetaminophen to potentiate its effect, such as metoclopramide (10 mg) or caffeine (either in beverages or as a tablet). Nonsteroidal anti-inflammatory drugs (NSAIDs), of which ibuprofen is the most studied, may also be used periodically in the first 20 weeks of gestation.
As gestation progresses, increasing consideration of potential risks versus benefits of the medication should occur because of potential effects of NSAIDs on platelet function, fetal renal function, and premature closure of the ductus arteriosus. If NSAIDs are utilized in the third trimester, many experts would caution against daily use and suggest regular testing of fetal well-being, including measurement of the amniotic fluid volume. The barbiturate butalbital is commonly used in conjunction with acetaminophen or caffeine in pregnancy but in our experience has a significant propensity for causing rebound headaches with frequent use as well as potential for withdrawal and neurodevelopmental issues in the neonate. We therefore recommend that, if used at all, this agent be used only infrequently. Lastly, narcotics, specifically opioids such as codeine, oxycodone, propoxyphene, and morphine, may be considered in refractory cases. Again, caution should be exercised in the prolonged use of any narcotic agents, due to the potential for rebound headaches and their addictive potential for both mother and fetus [8]. We see many patients with rebound headaches from daily use of butalbital or narcotics by well-meaning physicians and therefore instruct patients to try to never use narcotics to treat headaches for more than one day in a row or more than three times a month.
Nonsteroidal anti-inflammatory drugs, acetaminophen, and opioids all reduce pain associated with migraine; however, to abort a migraine as it is beginning, triptans (5-hydroxytryptophan (5-HT) agonists) can be used. These drugs include almotriptan, sumatriptan, rizatriptan, zolmitriptan and anratriptan. Currently, there is limited experience with triptan use during pregnancy, but for patients with symptoms refractory to first-line medications, sumatriptan use may be considered. Sumatriptan is an FDA category C drug and has not been associated with adverse pregnancy outcomes in humans; however, data are limited [9,10]. The sumatriptan registry has the largest number of cases with 7/183 pregnancies showing birth defects, which is consistent with the 2–4% background rate of congenital anomalies in the general population [11]. While some experts would not use these agents in pregnancy until further evidence is published, we believe that the data on sumatriptan suggest that this agent does have a role in second- or third-line treatment of headaches in pregnancy [12].
Another class of drugs used to abort migraines are the ergotamine derivatives. Ergot alkaloids (e.g. Cafergot®) have been helpful for nonpregnant patients but their role as potent vasoconstrictors precludes their use during pregnancy. These drugs are absolutely contraindicated in pregnancy (FDA category X) as they have been associated with increased uterine contractions resulting in abortion or decreased blood flow to the fetus leading to hypoxic sequelae [13].
For patients with a history of severe migraines or chronic, recurrent migraines >4 times per month, beta-blockers, calcium channel blockers and antidepressants can all be used prophylactically to reduce the onset of new migraines [14]. When appropriate, underlying conditions (i.e. hypertension, depression) should help guide the selection of which class of drugs is most appropriate.
Beta-blockers such as propranolol, 80–240 mg/d, and timolol, 20–30 mg/d, have been proven efficacious for the prevention of migraine [15]. Beta-blockers with intrinsic sympathomimetic activity (acebutolol, alprenolol, oxprenolol, pindolol) seem to be ineffective for the prevention of migraine [16]. The long-acting beta-blockers such as propranolol and atenolol are FDA class D due to the possible risk of intrauterine fetal growth restriction in the second and third trimesters. Beta-blockers also have the potential adverse effect of causing a beta-blockade in the fetus as well as the mother. Beta-blockade produces fetal hypoglycemia, bradycardia, hyperbilirubinemia, and can cause fetal growth restriction. However, these studies are largely among women with underlying hypertension, also a risk factor for growth restriction, and whether there truly is a cause for significant concern about the use of any beta-blocker in pregnancy is widely questioned [17]. In women refractory to conventional therapy, we support the use of prophylactic beta-blockers after discussing risks and benefits.
Tricyclic antidepressants, such as amitriptyline (US FDA Class C), nortriptyline (US FDA Class D), doxepin (US FDA Class C), are also effective for headache prophylaxis [16]. In the antidepressant class, amitriptyline, 30–150 mg/ d, has been more frequently studied than other antidepressants and is the only one with consistent support for efficacy in migraine prevention. Nortriptyline is a metabolite of amitriptyline and therefore likely has similar efficacy (and toxicities). Occasional reports have associated the therapeutic use of amitriptyline with congenital malformations; however, the bulk of the evidence indicates it is safe during pregnancy [18]. Additionally, because of the extensive experience with tricyclic antidepressants, amitriptyline and nortriptyline are preferred over other antidepressants for use in pregnancy. In patients who are depressed with severe migraine, a tricyclic antidepressant may treat both conditions; however, the addition of a newer atypical antidepressant may be needed. The use of some tricyclics have been associated with neonatal withdrawal symptoms; however, no significant long-term effects have been attributed to their use [19].
Calcium channel blockers such as nifedipine, nimodipine, cyclandelate, and verapamil seem to have modest effects in preventing the onset of migraines [20]. Nifedipine is the most studied of these agents in pregnancy, and verapamil is perhaps the next best studied. These calcium channel blockers seem to be safe in pregnancy as long as the patient’s blood pressure remains normal [21]. Magnesium may have some role both as an acute treatment for migraine (IV 1 g magnesium sulfate) and in prophylaxis (PO trimagnesium dicitrate 600 mg once daily [22]).
Anticonvulsants such as divalproex sodium and sodium valproate are efficacious in treating migraines in nonpregnant patients [23,24]. However, these drugs have been associated with an increased risk for congenital malformations, significant neurodevelopmental problems and fetal death and are therefore to be avoided in pregnant patients and in women who may become pregnant [25,26].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


