Materials and Methods
Detailed methods for the PARIS individual participant data systematic review and meta-analysis have been published previously. A brief description of the methodology relevant to the analysis presented here is outlined in the following text.
Search strategy
The Cochrane Pregnancy and Childbirth Review Group’s register of trials was searched up to December 2005 for relevant trials. This register is maintained by the regular searching of Medline, Embase, CENTRAL, and relevant journals by hand; PARIS trialists were also contacted for any further studies.
Eligibility criteria
Studies were included if they randomized women at risk of developing preeclampsia, gestational hypertension, or intrauterine growth restriction (based on previous or current obstetric factors or a preexisting medical disease) to receive 1 or more antiplatelet agents (eg, low-dose aspirin or dipyridamole) vs a placebo or no antiplatelet agent. Quasirandomized trials, and trials that included women who started treatment postpartum or had a diagnosis of preeclampsia at trial entry were excluded.
The analysis presented here includes women enrolled for primary prevention only (ie, women without gestational hypertension). Each potentially eligible study was assessed independently by at least 2 members of the IPD steering group, and any differences of opinion were resolved by discussion.
Four main outcomes were prespecified: preeclampsia (hypertension with new-onset proteinuria at or beyond 20 weeks’ gestation); death in utero or death of the baby before discharge from the hospital; preterm birth at less than 34 weeks’ gestation; and infant small for gestational age at birth (as defined by individual trialists).
Data extraction and analyses
Anonymized data for each of the prespecified variables were requested from trialists for each woman randomized. Data were supplied in a variety of formats, recoded as necessary, and checked for internal consistency, consistency with published reports, and missing items. Inconsistencies or missing data were discussed with relevant trialists and corrected when necessary.
Analyses included all women randomized and were based on an intention to treat. The analysis was restricted to trials with at least 80% of data available for that particular outcome. Outcomes were analyzed in their original trial and then these individual results combined in a meta-analysis to give an overall measure of effect.
A fixed-effect model was used, and the level of heterogeneity assessed with the I 2 statistic. Random-effects models were also run to test the robustness of results to the choice of model. We used a fixed-effect analysis in which the heterogeneity was low (I 2 ≤30%) and random-effects analysis in which the heterogeneity was high (I 2 >30%). Analyses were done using SCHARP software, version 4.0 (SAS Institute, Cary, NC), for the original IPD analyses and RevMan software (The Nordic Cochrane Centre, Copenhagen, Denmark) for the analysis presented here. χ 2 tests for interaction were performed to assess whether there were statistically significant differences ( P < .05) in the treatment effect between subgroups.
The protocol prespecified subgroup analysis based on gestational age at randomization at a gestation of <16 weeks, 16–19 weeks, 20–23 weeks, 24–27 weeks, and ≥28 weeks and stated that if the numbers were insufficient for any category, categories would be combined. For this analysis, a subgroup analysis based on gestation at randomization before 16 weeks, compared with at or after 16 weeks, was carried out for the 4 main outcomes prespecified in the PARIS protocol: preeclampsia, death of the baby, preterm birth before 34 weeks, and a small-for-gestational-age baby.
Results
Information about the quality and characteristics of the included trials and main findings of the IPD meta-analysis can be found in the previous publication. Results from the subgroup analysis based on gestational age at randomization before 16 weeks and at or after 16 weeks are presented in the following text.
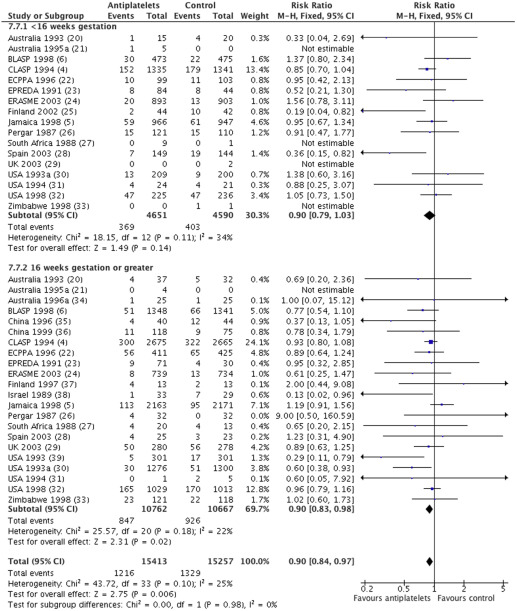
For the outcome of preeclampsia, individual participant data were available from 23 trials (30,670 women) with an overall relative risk of 0.90 (95% confidence interval [CI], 0.84–0.97) ( Figure 1 ). For women randomized before 16 weeks, the relative risk of preeclampsia was 0.90, with a 95% CI of 0.79–1.03 (17 trials, 9241 women). For those randomized at or after 16 weeks, the relative risk was also 0.90, with a 95% CI of 0.83–0.98 (22 trials, 21,429 women). There was no statistically significant difference in treatment effect between the 2 subgroups (interaction test, P = .98; heterogeneity I 2 , 25%).
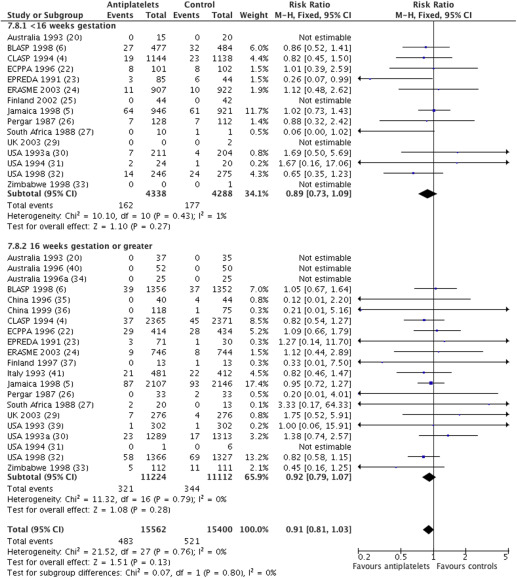
For the outcome death in utero or death of baby before discharge from the hospital, data were available from 22 trials (30,962 babies) with an overall relative risk of 0.91 (95% CI, 0.81–1.03) ( Figure 2 ). For women randomized before 16 weeks, the relative risk of death of the baby was 0.89, with a 95% CI of 0.73–1.09 (15 trials, 8626 women), and for those randomized at or after 16 weeks, it was 0.92 with a 95% CI of 0.79–1.07 (21 trials, 22,336 women). There was no statistically significant difference in treatment effect between the 2 subgroups (interaction test, P = .80; heterogeneity I 2 , 0%).
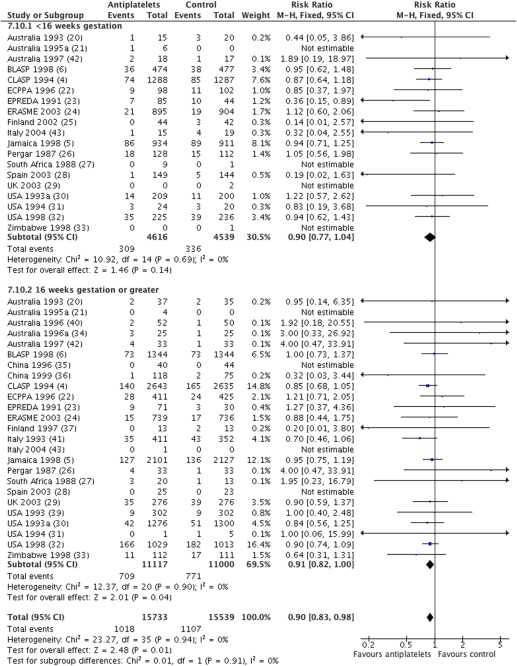
For preterm birth prior to 34 weeks’ gestation, data were available from 26 trials (31,272 women) with an overall relative risk of 0.90 (95% CI, 0.83–0.98) ( Figure 3 ). For women randomized before 16 weeks, the relative risk was 0.90, with a 95% of CI 0.77–1.04 (19 trials, 9155 women), and for those randomized at or after 16 weeks, it was 0.91, with a 95% CI of 0.82–1.00 (25 trials, 22,117 women). There was no statistically significant difference in treatment effect between the 2 subgroups (interaction test, P = .91; heterogeneity, I 2 , 0%).
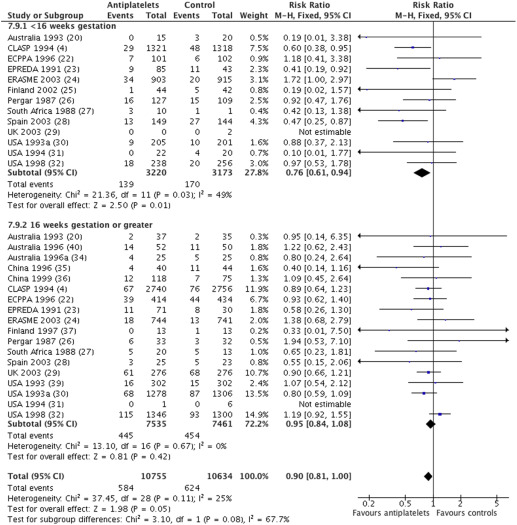
For the outcome of the baby being small for gestational age, data were available from 19 trials (21,389 women), with an overall relative risk of 0.90 (95% CI, 0.81–1.00) ( Figure 4 ). For women randomized before 16 weeks, the relative risk was 0.76, with a 95% CI of 0.61–0.94 (13 trials, 6393 women). For women randomized at or after 16 weeks, the relative risk was 0.95, with a 95% CI of 0.84 to 1.08 (18 trials, 14,996 women). There was no statistically significant difference in treatment effect between the 2 subgroups (interaction test, P = .08; heterogeneity I 2 , 25%).
Sensitivity analyses based on variations in the definitions of preeclampsia, a low vs a higher dose of aspirin (aspirin-only trials), and use of placebo were carried out for the main analysis. These factors did not have an impact on the overall results and therefore are unlikely to have an impact on the results of this subgroup meta-analysis ( Appendix Table 1 ).
A sensitivity analysis that included IPD data from antiplatelet trials published up to 2005 as well as aggregate data from trials published between 2005 and 2015 was conducted. This did not show any significant differences between subgroups whether women were randomised to antiplatelets before or after 16 weeks’ gestation for the 4 main outcomes ( Appendix Table 2 ). Although the risk of having a small-for-gestational-age baby was borderline for statistical significance (relative risk, 0.68 [95% CI, 0.50–0.92] for <16 week subgroup; relative risk, 0.95 [95% CI, 0.84–1.07] for >16 week subgroup; P = .05), these data should be interpreted with caution because women randomized at 16 weeks in some aggregate data studies could not be accurately placed in the appropriate subgroup for analysis.
Comment
This systematic review and individual participant data meta-analysis using data from more than 30,000 women found no difference in the risk of preeclampsia and its consequences, regardless of whether antiplatelet treatment was started before or after 16 weeks’ gestation, because the interaction tests for subgroup differences for all 4 outcomes were nonsignificant.
Strengths of our analysis are that each woman is correctly assigned to the appropriate subgroup, the data set is large, and potential for bias in selecting data for analysis is minimized. Because the outcome is rarely reported by gestation at randomization, the meta-analyses based on aggregate data may have assigned some participants to an incorrect subgroup.
Although published aggregate data meta-analyses include many of the studies included in the IPD meta-analysis (21 of 32 IPD studies by Roberge et al, 11 of 32 IPD studies by Xu et al ), in which women were recruited across a wide gestational age range, complete studies have been excluded from aggregate data subgroup analyses.
Overcoming these limitations using the individual participant data from each trial means that a key strength of our analysis is that it includes data for 9241 women from the 17 trials that recruited women before 16 weeks’ gestation and provided individual participant data for the original PARIS analysis, 6-fold more participants than reported in the previously published aggregate data analysis ( Table ).
| Review | Type of analysis | Number of trials | Number of women | Results for outcome preeclampsia RR (95% CI) | Subgroup interaction test |
|---|---|---|---|---|---|
| PARIS data set | Individual participant data meta-analysis | <16 wks: 17 ≥16 wks: 22 | <16 wks: 9241 ≥16 wks: 21,429 | <16 wks: RR, 0.90 (0.79–1.03) ≥16 wks: RR, 0.90 (0.83–0.98) a | No significant difference ( P = .98) a |
| Xu et al, 2015 | Aggregate data meta-analysis | ≤16 wks: 7 >16 wks: 14 | ≤16 wks: 1165 >16 wks: 3241 | ≤16 wks: OR, 0.37 (0.27–0.50) >16 wks: OR, 0.77 (0.62–0.97) | No significant difference ( P = .05) a |
| Roberge et al, 2013 b | Aggregate data meta-analysis | ≤16 wks: 13 >16 wks: 20 | ≤16 wks: 1479 >16 wks: 10,673 | ≤16 wks: RR, 0.47 (0.36–0.62) >16 wks: RR, 0.78 (0.61–0.99) | Significant difference ( P < .01) |
a Statistically significant result for individual subgroups but no statistically significant difference between subgroups
b Earlier version of this meta-analysis was published by the same authors, but data from the most up-to-date meta-analysis is presented here.
Reducing missing data reduces potential for bias. The aggregate data analysis also has a marked imbalance in the control group between the proportion of women who developed preeclampsia and were randomized before 16 weeks rather than after 16 weeks (17.9% and 8.4%, respectively). The incidence of preeclampsia in our individual participant data analysis also reflects that trial participants were women at an increased risk of preeclampsia; in the control group, there was no difference in the risk of preeclampsia for women recruited before 16 weeks rather than after 16 weeks (8.8% and 8.7%, respectively). Using individual participant data therefore reduced the potential to be misled either by the play of chance or by bias.
As for all subgroup analyses, this analysis by gestation at randomization is an indirect comparison and so should be interpreted with caution, as an observational analysis. Women were not randomized to early or late administration of antiplatelet treatment in these trials. The best test of whether findings of a subgroup analysis reflect a true difference or, as here, no difference is by confirmation in subsequent randomized trials. Nevertheless, the data presented here suggest that for aspirin and other antiplatelet agents, a randomized trial comparing the effects of starting treatment before or after 16 weeks may not be justified.
Subgroup analyses may be important to explore differential effects if they can be carefully justified. It is well known that aspirin may suppress aspects of endothelial dysfunction, but whether it also has an impact on placentation remains poorly understood. If the mechanism of action of aspirin in the prevention of preeclampsia is indeed related to placentation, it is not clear why a specific cutoff of 16 weeks should be used.
Placentation in early pregnancy is not completely understood, but it is believed that the first wave of trophoblast invasion is already complete around 10 weeks, the second wave does not start until 14–15 weeks, and active endovascular trophoblast has been seen up to 22 weeks. If aspirin reduces preeclampsia by its impact on endothelial dysfunction, this may explain why it is beneficial, even if given at later gestations.
Preterm birth prior to 34 weeks’ gestation was used as an indicator for early-onset or preterm preeclampsia in the PARIS Collaborative study, and there was no significant difference in this outcome between the subgroups. We have not reported the impact of gestation at randomization on severe preeclampsia because sufficient data are not available.
An aggregate data meta-analysis suggests that early aspirin is associated with a significant reduction in the risk of severe preeclampsia. These findings should be interpreted with caution because only a small proportion of eligible trials have reported the outcome severe preeclampsia ; therefore, findings are susceptible to reporting bias, and conclusions could potentially change significantly if all eligible studies had reported this outcome.
Searches to identify studies for the IPD meta-analysis were last updated in 2005. For the updated Cochrane Review, 7 new studies have been identified, which randomized women at or before 16 weeks’ gestation. These 7 studies were all small (totaling 705 women), and several had an unclear risk of bias and so are unlikely to change the overall conclusions of our analyses based on individual participant data for more than 9000 women randomized before 16 weeks.
A sensitivity analysis including IPD data and aggregate data from studies published between 2005 and 2015 showed no significant difference in overall results whether antiplatelets were given before or after 16 weeks’ gestation. However, the data should be interpreted with caution because the women randomized at 16 weeks in some aggregate data studies could not be accurately placed in the appropriate subgroup for analysis. Without obtaining IPD data from these studies, adding them to the IPD meta-analysis, is problematic and the findings are inconclusive.
Implications for clinical practice and research
Our analysis suggests that women at risk of preeclampsia should be offered antiplatelet therapy, usually low-dose aspirin, regardless of whether they are first seen before or after 16 weeks. It is unclear whether there is a gestational age beyond which commencing aspirin therapy may not be beneficial. Trials have recruited women after 28 weeks, and our prespecified IPD subgroup meta-analysis based on various gestational age cutoffs at randomization (<16 weeks, 16–19 weeks, 20–23 weeks, 24–27 weeks, and ≥28 weeks) found similar risk ratios across all subgroups ( Appendix Figure 1 ). Use before 12 weeks requires further evidence about safety, but aspirin can be commenced from 12 weeks with a reasonable reassurance of safety. Studies randomizing to aspirin therapy before 12 weeks may be indicated, particularly if earlier prediction of preeclampsia improves. Such studies should collect data on short- and long-term safety.
Conclusions
The effect of low-dose aspirin and other antiplatelet agents on preeclampsia and its complications is consistent, regardless of whether treatment is started before or after 16 weeks’ gestation. Women at an increased risk of preeclampsia should be offered antiplatelet therapy, even if health professionals first see them after 16 weeks’ gestation.
Acknowledgments
We thank the trialists who contributed data to the PARIS Collaborative Group. The PARIS Steering Group gave permission for the PARIS data set to be used for these analyses. Contribution to authorship included the following: L.A. and L.D. supplied the data from the PARIS Collaboration; L.A. and S.M. conducted the analysis; and S.M. and K.H. entered and checked the data. All authors contributed to and agreed to the manuscript. S.M. is the guarantor for the manuscript, and L.A. is the guarantor for the data from the PARIS Collaborative Group. Ethics approval was not required for this analysis because the IPD data used in the systematic review have been published previously. Funding for the original PARIS IPD analysis is reported elsewhere.
Appendix
| Characteristics | RR (95% CI) | Subgroup interaction test |
|---|---|---|
| Relative risk of preeclampsia in main analysis | 0.90 (0.84–0.97) | |
| PARIS definition of preeclampsia (SBP ≥140 mm Hg or DBP ≥90 mm Hg and trialist-defined proteinuria) | 0.90 (0.83–0.97) | Sensitivity analysis only |
| Trialists’ own definition of preeclampsia | 0.88 (0.81–0.96) | |
| Placebo-controlled studies | 0.90 (0.84–0.97) | P = .52 |
| No placebo | 0.71 (0.41–1.25) | |
| Aspirin dose ≤75 mg | 0.92 (0.85–0.99) | P = .23 |
| Aspirin dose >75 mg | 0.77 (0.61–0.97) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


