Antifungal Therapy
Patricia M. Flynn
Antifungal agents are available in systemic and topical formulations, and some are available in both forms. Topical antifungal agents, because they are poorly absorbed, are less likely to cause toxicity and should, as a rule, be the first choice for treating skin and mucous membrane infections. However, tinea capitis and onychomycosis (fungal infection of the nails) are best treated systemically (see Chapter 367). Infections that are severe, are disseminated, or involve the bloodstream should also be treated with systemic therapy. Organism- and disease-specific antifungal therapies are summarized in Table 247-1.
SYSTEMIC ANTIFUNGAL AGENTS
The most commonly used systemic antifungal agents can be divided into three major groups based on their structure and function: polyenes, azoles, and echinocandins.
 POLYENES
POLYENES
Amphotericin B is a polyene macrolide antimicrobial that is active against most fungal organisms, including Candida species, Cryptococcus neoformans, Histoplasma capsulatum, Blastomyces dermatitidis, Coccidioides immitis, and Aspergillus species. Amphotericin B is not active against Scedosporium species, some isolates of Fusarium species, Aspergillus terreus, and Candida lusitaniae. Amphotericin B acts by binding to sterols in the fungal cell membrane, causing increased cell permeability, leakage of cellular contents, and cell death. Current formulations include amphotericin B deoxycholate and three lipid formulations.
Amphotericin B deoxycholate is typically administered as a 1- to 6-hour (usually 2- to 4-hour) daily intravenous infusion of 0.6 to 1.5 mg/kg. The dosage is dependent on the infecting organism and the extent of infection. Amphotericin B deoxycholate is highly protein bound and accumulates in tissues, especially the liver and spleen. Thus, after therapy has been established, the drug can be administered every other day or 3 times weekly. The drug does not penetrate into the cerebrospinal fluid, vitreous humor, or amniotic fluid. Common side effects include infusion-related fever and chills that can be treated with meperidine, antipyretics, slowing the infusion rate, or a combination of these options.7 Patients who experience severe reactions can be given these agents or a hydrocortisone infusion before amphotericin B is administered. Neonates and children are less likely than adults to experience infusion-related toxicity. Nephrotoxicity (manifested by elevated serum creatinine and blood urea nitrogen concentrations), hypokalemia, and hypomagnesemia are also common, but usually reversible, side effects.7,12 Volume expansion with normal saline prior to administration of amphotericin B has reduced nephrotoxicity in adults.7
Lipid Formulations of Amphotericin B
Lipid formulations of amphotericin B result in less infusion-related and renal toxicities compared to amphotericin B deoxycholate.7,12 Thus, these agents can be given at higher doses than amphotericin B deoxycholate; however, nephrotoxicity and infusion-related symptoms remain the dose-limiting toxic effects. Three lipid formulations are now commercially available: amphotericin B lipid complex (Abelcet), liposomal amphotericin B (AmBisome), and amphotericin B colloidal dispersion (Amphotec). Each preparation achieves a higher concentration in the liver and spleen than does amphotericin B deoxycholate. Liposomal amphotericin B achieves higher peak concentrations in serum and brain than amphotericin B deoxycholate, and amphotericin B lipid complex reaches higher concentrations in the lung. Concentration of the parent drug in the kidney is similar for all of the lipid formulations of amphotericin B. Recommended daily dosages are variable and depend on the infecting organism and host susceptibility. Current recommendations are 5 mg/kg for amphotericin B lipid complex, 3 to 6 mg/kg for liposomal amphotericin B, and 3 to 4 mg/kg for amphotericin B colloidal dispersion. In general, most experts recommend 5 mg/kg of any lipid formulation for the treatment of serious fungal infections in pediatric patients.7,12
The lipid formulations are indicated for patients with aspergillosis who cannot tolerate amphotericin B deoxycholate therapy or for whom it has been unsuccessful. Amphotericin B lipid complex and liposomal amphotericin B are indicated for patients with other fungal infections if conventional therapy has failed or caused toxic effects. Liposomal amphotericin B is also indicated for the empiric treatment of febrile neutropenic cancer patients, cryptococcal meningitis in HIV-infected patients, and for patients with visceral leishmaniasis.
Despite the much higher cost of the lipid formulations and the necessity for higher dosages, utilization of these agents is increasing in pediatric patients because of their improved safety profile.
 AZOLES
AZOLES
The available systemic azoles include the imidazoles, miconazole and ketoconazole, and the triazoles, fluconazole, itraconazole, voriconazole, and posaconazole. Because they are less toxic, the triazoles have largely replaced miconazole as treatment for systemic fungal infections. The introduction of second-generation triazoles (voriconazole and posaconazole) has greatly expanded the range of susceptible organisms and use of azoles. These agents are fungistatic. In general, the azole family of drugs has activity against Candida albicans and the dimorphic fungi H capsulatum, B dermatitidis, C immitis, and C neoformans. Some non-albicans species of Candida are also susceptible, especially to the second-generation triazoles, as are the dermatophytes. Itraconazole, voriconazole, and posaconazole have activity against Aspergillus species. Voriconazole and posaconazole have activity against Scedosporium apiospermum and Fusarium species and many isolates of Candida spp that are resistant to fluconazole. Posaconazole has activity against zygomycetes. The azoles act by inhibiting sterol 14-α-demethylase, a cytochrome P450 enzyme system; this inhibition results in impaired ergosterol synthesis in the fungal cell membrane.7,12 When an azole is selected for systemic use, possible drug interactions should be carefully considered (eTable 247.1  ).
).
Ketoconazole
This drug is effective therapy for candidiasis, chronic mucocutaneous candidiasis, blastomycosis, histoplasmosis, coccidiodomycosis, chromomycosis, and paracoccidioidomycosis. It is available as an oral suspension, a tablet, a shampoo, and a topical cream. It is readily absorbed from the gastrointestinal tract and can be administered to children every 12 to 24 hours at a total daily dosage of 5 to 10 mg/kg. Ketoconazole is highly protein bound and does not penetrate the central nervous system (CNS). Gastrointestinal distress, the most frequent side effect, can be reduced if patients take the medication with food. The most severe adverse effect is rare fatal hepatotoxicity. Of all the orally absorbed azoles, ketoconazole has the greatest effect on the host endocrine system. It has the same potential serious interaction with terfenadine, cisapride, and astemizole as does itraconazole.
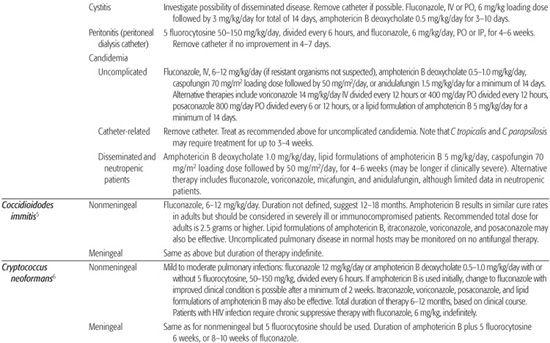
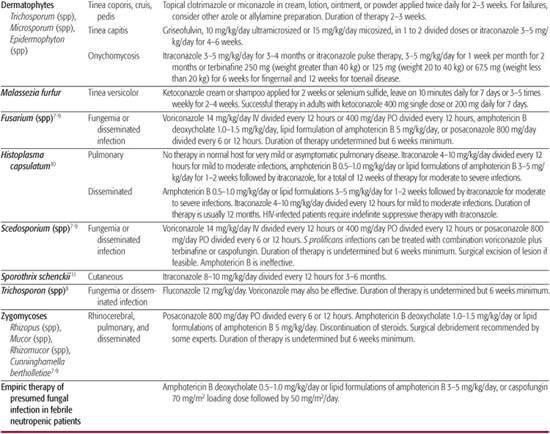
Table 247–1. Antifungal Therapy
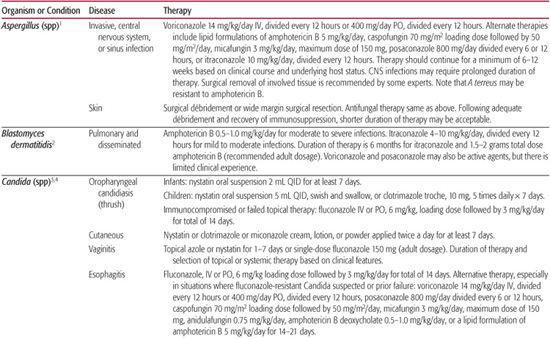
Fluconazole
This drug is effective for the treatment of esophageal, laryngeal, and vaginal candidiasis. It should also be considered in cases of oropharyngeal candidiasis unresponsive to topical therapy. Fluconazole (Diflucan) can be used to treat serious candidal infections in nonneutropenic, hemodynamically stable patients unless the infection is suspected to be caused by a resistant strain such as Candida krusei, guillermondii, or glabrata.3,4 The drug has also been used for prophylaxis of fungal infection in adult and pediatric recipients of hematopoietic stem cell transplants, solid organ transplants, and in premature infants.13,14 Fluconazole can be used as initial therapy for mild cases of cryptococcal meningitis in adults with acquired immunodeficiency syndrome (AIDS) and for long-term suppression of cryptococcal infection. Its usefulness in treating patients with non-AIDS–associated cryptococcal meningitis has not been defined. Fluconazole is also effective in coccidioidal meningitis and other types of coccidioidomycosis.
Fluconazole is available as an oral suspension, a tablet, and an intravenous solution. It is well absorbed from the gastrointestinal tract, and the recommended oral and intravenous dosages are identical. For patients with oropharyngeal candidiasis, a loading dose of 6 mg/kg, followed by 3 mg/kg daily for 2 weeks, is recommended. The recommended daily dosage for patients with systemic fungal infection is 400 to 800 mg for adults and approximately 6 to 12 mg/kg for children. Some experts recommend that premature infants should receive the same dosage as older children, but the drug should be administered only once every 72 hours for the first 2 weeks of life and once every 48 hours for the subsequent 2 weeks of life.12 Others recommend every other day dosing during the first week of life in this population.7 Fluconazole readily enters body fluids, including the cerebrospinal fluid. The drug is excreted unchanged in the urine, and the dosage must be adjusted for patients with renal impairment. The most common side effects are gastrointestinal complaints; less frequently, hepatic enzyme activity may be elevated. Development of resistance to fluconazole is reported with increasing frequency. Failure of fluconazole treatment may be attributable to resistance, especially in patients who have received chronic suppressive therapy, and to the drug’s fungistatic, rather than fungicidal, effects.
Itraconazole
This agent has activity similar to that of fluconazole but is also active against Aspergillus species and certain dematiaceous molds. It is indicated for the treatment of blastomycosis, histoplasmosis, aspergillosis, and onychomycosis. Recent studies show it to be effective in tinea capitis in adults and children. Itraconazole (Sporanox) has also been used for the prophylaxis and empiric therapy of fungal infections in neutropenic cancer patients and hematopoietic stem cell transplant recipients.15,16 The oral solution of itraconazole is effective therapy for oropharyngeal and esophageal candidiasis. Currently, the agent is available as a capsule and an oral solution. The preparations are not interchangeable; the oral solution produces greater systemic drug exposure than the equivalent dose in capsule form. Because bioavailability is affected by the acidity of the stomach, the capsules should be taken with food. The oral solution is less affected by stomach pH and should be taken while the patient is fasting.12 Itraconazole is highly protein bound and very little penetrates into the cerebrospinal fluid. It is metabolized by the liver and excreted as inactive metabolites in the feces and urine. Although neither formulation is approved by the US Food and Drug Administration for use in children, reports of multiple pediatric studies describe the administration of itraconazole at dosages as high as 10 mg/kg per day for severe infections and 3 to 5 mg/kg per day for tinea capitis and onychomycosis.7,17 The duration of therapy for onychomycosis is 3 to 4 months. However, because the drug accumulates in the nail tissues, “pulse therapy” (repeated courses of 1 week of therapy followed by 1 to 3 weeks without therapy) has been reported effective for onychomycosis.17 As with fluconazole, gastrointestinal side effects occur most frequently. Use of itraconazole with terfenadine, cisapride, or astemizole can cause a serious drug interaction that produces life-threatening cardiac arrhythmias. Itraconazole has been reported to potentiate vincristine toxicity in children undergoing concurrent chemotherapy for leukemia.
Voriconazole
Voriconazole (VFend), a second-generation triazole, is approved for use in the treatment of invasive aspergillosis, serious Candida species infections in nonneutropenic patients, including candidemia, disseminated disease and infections caused by fluconazole-resistant species, and for serious infections due to S apiospermum and Fusarium species in patients refractory to or intolerant of other therapy. Voriconazole was demonstrated to be superior to amphotericin B deoxycholate in the initial treatment of aspergillosis.1 Published reports suggest voriconazole also demonstrates activity in a variety of less-common or refractory fungal infections.7-9 Voriconazole is not active against the zygomycetes, and clinical reports suggest emergence of these infections following prophylactic administration of voriconazole.
Voriconazole is available as an intravenous preparation, tablet, and oral suspension. Children require higher doses of voriconazole than adults. The most recent recommendations are that children, ages 1 to 11 years, receive an intravenous dosage of 7 mg/kg every 12 hours and an oral dosage of 200 mg of the oral suspension every 12 hours without loading doses. Serum concentrations can be obtained to support further modification of pediatric dosing.7 Voriconazole concentrations in tissue and cerebrospinal fluid (CSF) exceed those of the trough plasma level severalfold.
Visual disturbances, dermatologic reactions, and elevated liver transaminases are the most common adverse effects associated with voriconazole administration. Visual disturbances include blurred vision, photophobia, and altered or enhanced visual perception and occur in approximately 25% to 45% of recipients. These reactions typically occurred within 30 minutes of drug administration and had a median duration of 30 minutes. Rash, including photosensitivity, occurs in less than 10%. Increased liver transaminases occur in approximately 10% to 20% of recipients. For patients with mild to moderate hepatic insufficiency (Child-Pugh Class A and B scores 5 to 9), the manufacturer recommends giving the normal loading dose but halving the maintenance dose. There are no data available on administration in patients with more severe hepatic dysfunction. Dose adjustments for patients with renal insufficiency are not necessary for the oral formulation. However, administration of the intravenous form should be avoided in patients with moderate to severe renal impairment due to the formulation of the product. Dose adjustment is not routinely indicated for patients on dialysis.
Posaconazole
Posaconazole (Noxafil) has even broader coverage than voriconazole with activity against the zygomycetes. Currently posaconazole is indicated for the treatment of oropharyngeal candidiasis, including refractory cases, and for prophylaxis of fungal infection in patients older than 13 years undergoing hematopoietic stem cell transplant or with prolonged neutropenia. In addition, posaconazole has demonstrated efficacy as salvage therapy in a variety of fungal infections, including aspergillosis, fusariosis, and zygomycosis.
Posaconazole is currently available only as an oral suspension. Absorption of the suspension is improved with divided daily dosing and high-fat meals. The recommended dose for adults for prophylaxis is 200 mg 3 times daily. For treatment of serious fungal infections, doses of 800 mg daily, divided 2 to 4 times daily, are used. There are limited pharmacokinetic data for posaconazole in children. A small number of older children, age 8 to 17 years, demonstrated similar concentrations as in adults, and the adult dose is recommended in children.
Posaconazole is generally well tolerated; gastrointestinal symptoms, including vomiting, abdominal pain, and diarrhea, are the most commonly reported side effects.
 ECHINOCANDINS
ECHINOCANDINS
The echinocandins are the most recently discovered class of antifungals. They act by inhibiting the synthesis of 1,3-beta-D-glucan, a major component of the fungal cell wall. Because there is no counterpart in the mammalian cell, the echinocandins are predictably much better tolerated compared to other antifungal agents. Currently, three echinocandins are commercially available as intravenous formulations and have the same general spectrum of activity. They have long half-lives and can be dosed once daily. Echinocandins are fungicidal against Candida species, including azole-resistant species, and fungistatic against Aspergillus species and not active against Cryptococcus, Fusarium, or zygomycetes.7
Caspofungin
Caspofungin (Cancidas) is approved for treatment of esophageal candidiasis, candidemia, other candidal infections, treatment of aspergillosis in patients who are refractory to or intolerant of other therapies, and as empiric therapy for presumed fungal infections in febrile, neutropenic patients. The agent is highly protein bound and penetrates into all major organs including the brain. However, concentrations in uninfected CSF are low, and there is a lack of clinical data to support its use in CNS infections.7
Caspofungin is available as an intravenous formulation only. Recommended dosage for children is a loading dose of 70 mg/m2 followed by daily administration of 50 mg/m2. No dose adjustment is indicated for patients who are on dialysis or have renal or mild hepatic insufficiency. Reduction in the daily dose from 50 mg to 35 mg is recommended for adults with moderate hepatic insufficiency (Child-Pugh class B, or score 7 to 9). There is no information on administration in patients with severe hepatic dysfunction.
The most common adverse events reported with the use of caspofungin are mild increases in hepatic transaminases, gastrointestinal upset, and headache.
Micafungin
Micafungin (Mycamine) is indicated for the treatment of esophageal candidiasis and as prophylaxis in patients undergoing stem cell transplant. In addition, there are clinical studies demonstrating efficacy in disseminated candidiasis and in invasive aspergillosis in patients refractory to or intolerant of other antifungal therapy. Micafungin is highly protein bound and achieves highest tissue concentrations in the lung. Low levels can be detected in the brain, and levels were undetectable in CSF.12
Micafungin is available as an intravenous preparation and has been studied in children and neonates. The drug was well tolerated without dose-related toxicities. Recommended pediatric dosing for children older than 2 years is 1 mg/kg/day (maximum dose of 50 mg daily) for prophylaxis and 3 mg/kg/day (maximum dose of 150 mg daily) for treatment. In salvage treatment studies of aspergillosis, children received doses up to 7 mg/kg/day safely. Premature infants less than 1000 g have a shorter half-life and more rapid clearance, and a dose of 8 to 10 mg/kg/dose once daily is currently under investigation. For neonates > 1000 g, 5 to 7 mg/kg/dose once daily is currently under investigation. For infants and children younger than 2 years, 6 mg/kg/dose once daily is recommended. Patients with renal insufficiency and mild or moderate hepatic dysfunction do not require dose adjustments of micafungin.
Anidulafungin
Anidulafungin has a similar spectrum of activity and safety profile compared to other echinocandins. Animal data indicate a large volume of distribution, suggesting extensive tissue distribution. The half-life is even longer than that of the other echinocandins, greater than 24 hours in adults.7 Anidulafungin is indicated for the treatment of esophageal candidiasis and candidemia and other forms of Candida infections (intra-abdominal abscess and peritonitis) in nonneutropenic patients. Tissue concentrations are highest in the lung and liver. Measurable concentrations were detected in the brain.
The recommended dosing for adults is a loading dose of 200 mg followed by a 100 mg daily dose for systemic Candida infections and a 100 mg loading dose followed by 50 mg daily for esophageal candidiasis. The pediatric equivalent is approximately 1.5 mg/kg/day and 0.75 mg/kg/day, respectively. Dose adjustment is not needed for renal insufficiency and mild, moderate, or severe hepatic insufficiency.
Anidulafungin is well tolerated with gastrointestinal symptoms and mild increases in hepatic transaminases as the most commonly attributed events.
 OTHER SYSTEMIC ANTIFUNGAL AGENTS
OTHER SYSTEMIC ANTIFUNGAL AGENTS
Flucytosine
Flucytosine (5-FC), a fluorinated pyrimidine related to fluorouracil, is indicated for treating serious infections caused by Candida species and C neoformans. It is converted to fluorouracil within fungal cells, where it interferes with RNA and DNA synthesis.12 Flucytosine is available in capsule form and is readily absorbed from the gastrointestinal tract. Because the drug is poorly protein bound and penetrates the blood-brain barrier, it is effective as adjunctive therapy for meningitis, in combination with amphotericin B. The recommended daily dosage is 50 to 150 mg/kg, divided into 4 equal doses. The dosage must be adjusted for patients with renal impairment, and caution should be used when flucytosine is administered to patients with underlying renal dysfunction. Gastrointestinal complaints, hepatitis, and jaundice are frequent adverse effects. Plasma drug concentration should be maintained at ≤ 40 to 80 µg/mL to avert bone marrow suppression, which is the most serious toxicity.12 Flucytosine should be used in combination with other anti-fungal agents, such as amphotericin B, because pathogens rapidly acquire resistance to flucytosine when it is used alone.
Griseofulvin
Griseofulvin is indicated for treatment of superficial fungal infections of skin, hair, and nails that are caused by various species of dermatophytes, including Trichophyton, Microsporum, and Epidermophyton. It acts by inhibiting fungal cell mitosis at metaphase and by binding to human keratin. Absorption from the gastrointestinal tract is markedly variable but is thought to be increased by fatty meals and by the use of smaller griseofulvin crystals. The daily dosage for children is approximately 10 mg/kg of ultramicrosized and 15 mg/kg of microsized griseofulvin, given as 1 dose or 2 divided doses. Tinea corporis requires 2 to 4 weeks of therapy, tinea capitis 4 to 6 weeks of therapy, and infection of toenails at least 6 to 12 months of therapy. The safety of griseofulvin treatment is not established for children younger than 2 years of age. Griseofulvin is usually well tolerated; rash and urticaria are the most common adverse effects. Headache is also common. 
Terbinafine
Terbinafine is an allylamine that acts by inhibiting fungal synthesis of ergosterol. It has been available as a topical agent for a number of years, but has only recently become available in an oral tablet form for treatment of onychomycosis. Allylamines act by inhibiting the epoxidation of squalene, thus blocking the biosynthesis of ergosterol, a component of the fungal cell membrane. The drug has not been tested in children and should be reserved for patients with refractory disease. The recommended adult regimen is 250 mg daily for 6 weeks for fingernail disease and daily for 12 weeks for toenail disease. Children who weigh more than 40 kg should receive the adult dosage. The dosage should be 125 mg/d for children who weigh between 20 and 40 kg, and 62.5 mg/d for children who weigh less than 20 kg. Gastrointestinal toxicity is observed most frequently.
COMBINATION SYSTEMIC THERAPY
As multiple classes of systemic antifungal agents are now available, there is considerable interest in combining agents for the treatment of serious invasive fungal infections. To date, the efficacy of combination of amphotericin B deoxycholate plus 5-flucytosine in cryptococcal meningitis and amphotericin B deoxycholate plus fluconazole in candidemia remain the only combinations supported by evidence from randomized clinical trials. Furthermore, some data from animal models suggest that the combination of amphotericin B plus an azole can be antagonistic. Until there is further documentation of the increased efficacy of combination anti-fungal therapy (other than those currently supported by clinical trials), it should be restricted.
TOPICAL ANTIFUNGAL AGENTS
Topical antifungal agents are available in many different preparations, including ointment, cream, solution, lotion, powder, oral and vaginal troches, and vaginal tablets. Topical agents are the treatment of choice for superficial fungal infections of the skin, mucosa, and cornea, such as dermatophytosis (tinea corporis, tinea cruris, tinea pedis), tinea versicolor, candidiasis, and fungal keratitis. Creams and lotions are generally preferable to ointments for treating diseases of the skin. Powders are used only in moist areas such as the feet, groin, or other intertriginous areas. Several of the agents used as systemic therapy are also available as topical preparations. The mechanism of action is the same, but the efficacy of the topical agents depends on their direct interaction with the fungal organisms on the surface of the skin or mucous membranes. In selecting a topical antifungal agent, consideration should be given to the type of preparation needed, the cost, and its bioavailability. Several agents now available without prescription may be used as first-line therapy for tinea corporis, with more expensive preparations reserved for resistant infection.
 POLYENE ANTIFUNGALS
POLYENE ANTIFUNGALS
Nystatin
The mechanism of action of nystatin is similar to that of amphotericin B, but nystatin is active only against superficial candidiasis. Nystatin suspension is the treatment of choice for oral candidiasis and should be administered 4 times daily. Children who are able should be instructed to swish the suspension around the mouth and then swallow. The side effects include nausea and bad taste, but both are manageable. Topical nystatin is also frequently used for candidiasis in the diaper area and is available in combination with corticosteroids. Nystatin powder is effective for treating superficial candidiasis in skin folds, but care should be taken to prevent young infants’ inhalation of the powder when it is applied to their necks.
 AZOLES
AZOLES
Multiple azole antifungal agents are available as topical preparations. They are active against Candida species, Trichophyton species, Microsporum spp, and, in some cases, Malassezia furfur. The mechanism of action is that described for systemic azoles. When used as directed, topical azoles cause few side effects. Some redness and irritation can occur.
Clotrimazole is available as an oral troche, a vaginal tablet, a vaginal cream, cream, and a lotion. Miconazole is also available over the counter as a cream, spray, powder, lotion, and vaginal cream. These agents are readily available and inexpensive, and they should be the treatment of choice for tinea corporis (including tinea pedis and tinea cruris) and vaginal candidiasis. Topical ketoconazole, econazole, sulconazole, sertaconazole, and oxiconazole are also available. These agents have a similar spectrum of activity but should be considered second-line agents because of cost. Ketoconazole is available as a shampoo for treatment of tinea versicolor but is ineffective therapy for tinea capitis. Terconazole, butoconazole, and tioconazole are available only as vaginal preparations.
 ALLYLAMINES
ALLYLAMINES
Three topical allylamine agents—terbinafine, naftifine, and butenafine—are currently available. As a group, allylamines are active against the dermatophytes. These agents have not been studied in children younger than 12 years of age.
 OTHER TOPICAL ANTIFUNGAL AGENTS
OTHER TOPICAL ANTIFUNGAL AGENTS
Tolnaftate is active against dermatophytes but is ineffective against Candida species and is less effective than the azoles for treating tinea corporis. Many preparations of tolnaftate are available without prescription. Ciclopirox is a topical antifungal with broad-spectrum activity against the dermatophytes, including M furfur.
REFERENCES
See references on DVD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


