Chapter 7
Anesthesia for Fetal Surgery and Other Intrauterine Procedures
Mark D. Rollins MD, PhD, Mark A. Rosen MD
Chapter Outline
INDICATIONS AND RATIONALE FOR FETAL SURGERY
Anesthesia for Minimally Invasive and Percutaneous Procedures
Fetal therapy originated in 1963 with Sir William Liley’s successful intraperitoneal blood transfusion to a fetus with erythroblastosis fetalis.1 This was followed by many years of discouraging attempts to transfuse blood via direct cannulation of fetal vessels through a small uterine incision.2 In 1981, after careful experimentation and practice in sheep3,4 and rhesus monkeys,5 the first successful human fetal surgery, a vesicostomy, was performed in a fetus with bilateral hydronephrosis due to a lower urinary tract obstruction.6
Advances in prenatal diagnostic technology, particularly in the resolution of imaging, contribute to increasing sophistication in diagnosis of fetal disorders, principally anatomic anomalies. Fetal therapy is largely nonsurgical (e.g., administration of medications, nutrients, blood, stem cells) (see Chapter 6). Some identified disorders are amenable to intrauterine fetal surgery, but most anatomic malformations diagnosed in utero remain unsuitable for antenatal intervention. Prenatal diagnosis of serious malformations (e.g., those that are uncorrectable and incompatible with normal postnatal life) allows the choice of pregnancy termination. Most correctable malformations are best managed after delivery at term gestation, but antepartum recognition allows time for the coordination of appropriate prenatal and postnatal care, including transfer of peripartum care to an appropriate medical center while the fetus is in utero rather than as a newly delivered, fragile neonate. Some defects, especially those that cause airway obstruction, can be treated with an intrapartum intervention, in which the fetus undergoes repair of the defect and/or the airway is secured during birth, while the uteroplacental unit remains functional.
Fetal surgery is reasonable only with informed consent and only if (1) the lesion is diagnosed accurately, (2) the lesion’s severity is assessed correctly, (3) the associated anomalies that contraindicate intervention are excluded, (4) the maternal risk is acceptably low, and (5) the neonatal outcome would be better with in utero surgery than with surgery performed after delivery. With all fetal surgical procedures, an emphasis also must be placed on maternal welfare to guard against undue maternal risk.7,8
Fetal surgical interventions can be broadly categorized into three kinds of procedures, namely, open surgical procedures, minimally invasive procedures, and intrapartum procedures. Open surgical procedures involve both maternal laparotomy and hysterotomy with use of pharmacologic agents to maintain uterine relaxation. These procedures are typically performed near mid gestation and entail greater maternal and fetal risks compared with the minimally invasive techniques, including a significant risk for preterm premature rupture of membranes (PROM), preterm labor and delivery, uterine dehiscence, oligohydramnios, hemorrhage, pulmonary edema, and fetal mortality.9,10 In addition, after an open surgical procedure, a cesarean delivery is required for the subsequent delivery and all future deliveries owing to the location of the hysterotomy and the associated risks for uterine dehiscence or rupture. Open fetal surgical procedures have been used to repair fetal myelomeningoceles, resect congenital pulmonary airway abnormalities, and debulk sacrococcygeal teratomas.
The third kind of procedure involves a modification of cesarean delivery to allow intrapartum fetal therapy while the fetus remains supported by placental gas exchange. These delivery techniques are termed EXIT procedures, for ex utero intrapartum therapy.11 EXIT procedures are most often employed (1) to secure the airway by endotracheal intubation, bronchoscopy, or tracheostomy or (2) to perform other fetal procedures while gas exchange continues in the placenta (placental bypass). The EXIT procedure enables the prevention of postnatal asphyxia in newborns with lesions such as cystic hygroma, lymphangioma, cervical teratoma, and congenital syndromes, in whom securing an airway after birth can be problematic. The procedure is also used as a bridge to extracorporeal membrane oxygenation (ECMO) for a fetus with cardiopulmonary disease at risk for postnatal cardiac failure or failure of adequate gas exchange in the lungs.
Fetal surgery is a reasonable therapeutic intervention for certain correctable fetal anomalies with predictable, life-threatening, or serious developmental consequences. If untreated, these lesions can interfere with fetal organ development or result in cardiac failure; if corrected in utero, irreversible organ damage and fetal demise may be prevented.
Indications and Rationale for Fetal Surgery
Bilateral Hydronephrosis–Obstructive Uropathy
Congenital obstructive uropathy occurs in approximately 0.1% of pregnancies.12 Congenital bilateral hydronephrosis results from fetal urethral obstruction at the bladder outlet, most often by posterior urethral valves in male fetuses or urethral obstruction in females. Other causes of fetal obstructive uropathy include obstruction at the ureteropelvic or vesicoureteric junction and a number of complex disorders in females (e.g., cloacal plate anomalies). These uropathies are easily detected by ultrasonography, which is often performed to investigate oligohydramnios from diminished fetal urine output. Severe obstructive lesions may lead to progressive renal dysplasia and dysfunction, bladder distention, and oligohydramnios and ultimately result in devastating developmental consequences, such as limb and facial deformities and pulmonary hypoplasia (Figure 7-1).13 Preterm delivery allows early urinary tract decompression ex utero, but fetal pulmonary immaturity limits the efficacy of this approach. Early intrauterine intervention with placement of a vesicoamniotic shunt allows drainage of urine from the fetal bladder into the amniotic cavity, thereby decompressing the urinary tract. In animal models, in utero relief of obstructive uropathy improves dysplastic renal histology, restores normal urine flow and amniotic fluid volume, and results in improved lung growth and development.14 The applicability of these findings to human fetal obstructive uropathy remains unclear and controversial.15
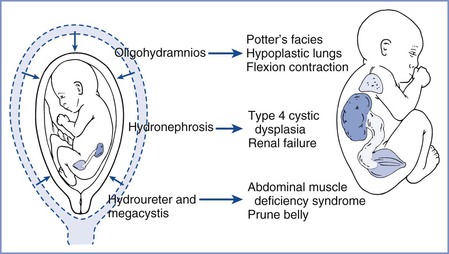
FIGURE 7-1 Developmental consequences of fetal urethral obstruction. Obstructed fetal urinary flow results in hydronephrosis, hydroureter, megacystis, oligohydramnios, and pulmonary hypoplasia. (Redrawn from Harrison MR, Filly RA, Parer JT, et al. Management of the fetus with a urinary tract malformation. JAMA 1981; 246:635-9.)
Vesicoamniotic catheter shunts have been used for intrauterine treatment of bilateral hydronephrosis since the early 1980s.16 These valveless, double-coiled catheters are placed percutaneously with ultrasonographic guidance, with one coil being left in the urinary bladder and the other in the amniotic space. Common problems associated with these catheters include (1) difficult placement, occlusion, and displacement; (2) fetal trauma, iatrogenic abdominal wall defects, and amnioperitoneal leaking; and (3) preterm PROM, preterm labor, and infection.17 Neonatal survival rates after fetal vesicoamniotic shunting (performed from the 1980s to 2001) varied from 50% to 90%, with approximately half of the survivors having normal renal function.15,18,19 Currently a multicenter, randomized controlled trial is underway comparing the perinatal mortality and renal function of fetuses with lower urinary tract obstruction treated by either vesicoamniotic shunting or conservative noninterventional care.20
Fetal cystoscopy is a more recent treatment option that allows direct visualization of the fetal urethra. Although not a viable treatment for urethral atresia, fetal cystoscopy facilitates diagnosis and treatment of lower urinary tract obstruction due to posterior urethral valves.21 Fetal cystoscopy with ablation of posterior urethral valves appears to provide a survival advantage over conservative therapy but has not been demonstrated to improve perinatal survival over vesicoamniotic shunting.22
Current evidence supports fetal surgery for the correction of obstructive uropathy in selected fetuses in an effort to restore amniotic fluid volume, prevent pulmonary hypoplasia, and decrease perinatal mortality. However, the effects on long-term renal function and other morbidities remain unclear, and additional evidence is needed.
Congenital Diaphragmatic Hernia
Approximately 1 of 2500 live newborns has a congenital diaphragmatic hernia (CDH).23 Without fetal intervention, this anomaly causes significant mortality from pulmonary hypoplasia and insufficiency. Survival rates have improved to between 60% and 85% over the past 20 years24–27 and are closely associated with the degree of pulmonary hypertension and dysfunction.24 Significant mortality occurs despite optimal postnatal surgical management at tertiary care medical centers (i.e., procedures involving removal of the herniated viscera from the chest, administration of surfactant, ventilation techniques that minimize lung trauma, use of ECMO, and closure of the diaphragm). Intrauterine correction of CDH has the potential to prevent the development of pulmonary hypoplasia and allow the fetal lung to develop before delivery.
The use of a fetal lamb model demonstrates that parenchymal hypoplasia and associated pulmonary vascular changes can be reversed by correction in utero.3 Primary repairs of human CDH in utero have been undertaken only for fetuses with severe disease, with limited success but many lessons learned, including the development of minimally invasive approaches.28,29
Fetal lungs contribute to amniotic fluid volume by secreting more than 100 mL/kg/day of fluid that exits the trachea and mouth. Tracheal occlusion impedes the normal egress of fetal lung fluid and results in expansion of the hypoplastic lung, thereby inducing lung growth and cellular maturation in fetuses with CDH.30,31 This occlusion technique, termed “plug the lung until it grows” (i.e., PLUG),32,33 replaced primary repair in utero for the correction of the pulmonary hypoplasia associated with CDH. It is a less extensive, palliative fetal surgical procedure that enhances lung growth to improve postnatal survival, with postponement of the definitive repair until after birth.30,34 Once the trachea is occluded, fetal pulmonary fluid slowly accumulates and expands the lung, pushing the viscera out of the thorax. A small detachable balloon for endoluminal tracheal occlusion is placed in the trachea via percutaneous endoscopic endotracheal intubation and is either left in place until delivery or deflated and removed earlier (Figure 7-2).35,36
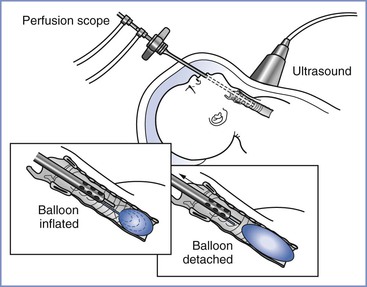
FIGURE 7-2 Schematic of fetal tracheal occlusion using a balloon. (From Harrison MR, Albanese CT, Hawgood SB, et al. Fetoscopic temporary tracheal occlusion by means of detachable balloon for congenital diaphragmatic hernia. Am J Obstet Gynecol 2001; 185:730-3.)
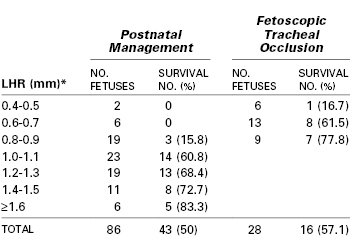
A prospective randomized trial (1999-2001) evaluated fetal tracheal occlusion for intrauterine treatment of severe CDH.37 Inclusion criteria included (1) a gestational age of 22 to 28 weeks, (2) left-sided herniation of the liver into the hemithorax, and (3) a low lung-to-head ratio (LHR) (i.e., < 1.4). The LHR is a ratio of the contralateral lung size compared with head circumference and is correlated with the severity of pulmonary hypoplasia and survival for a given gestational age.38 The trial was closed early (n = 11); fetal tracheal occlusion resulted in no improvement in survival compared with control (77% versus 73%) and no reduction in morbidity at 90 days. The rates of preterm PROM and preterm delivery were higher in the fetal intervention group.37 However, the survival rate was unexpectedly high in the control group. It is possible that the LHR criterion of less than 1.4 was not sufficiently restrictive and allowed inclusion of fetuses in the study that were likely to survive with standard postnatal tertiary medical care. Table 7-1 notes outcomes of left-sided CDH fetuses treated in utero or with standard postnatal care.
More recently in Europe, the Fetal Endoscopic Tracheal Occlusion (FETO) Task Force began a collaboration among three medical centers for treatment of severe cases of CDH with a high risk for death.39 FETO intervention criteria for fetuses at high risk included both LHR less than 1.0 and liver herniation into the hemithorax.40 Use of smaller-gauge endoscopes and reversal of the tracheal occlusion before birth appear to show great promise for reduction in the risk for preterm delivery due to preterm PROM.41,42 Owing to concern for tracheal damage by very early tracheal balloon placement,43 the tracheal balloon is placed between 26 and 28 weeks’ gestation and removed before birth by a second fetoscopic procedure near 34 weeks (if the fetus is still in utero).44 This second procedure is performed to minimize the risk of preterm labor, avoid the need for the EXIT procedure, and potentially improve lung growth and minimize the reduction of type II alveolar cells associated with prolonged tracheal occlusion. Outcomes for 210 cases (through 2008) of fetuses with a mean gestational age of 27 weeks, LHR less than 1.0, and primarily left-sided CDH (84%) were compared with those for historic postnatal treatment controls (1995-2004). Use of FETO significantly improved the survival rate (47% versus 20%), and delivery occurred at a median gestational age of 35.3 weeks.39 However, the comparative results may represent selection bias or improvement in technique and clinical care over time. A more recent (2008-2010) randomized, controlled, single-institution trial compared cases of severe CDH (LHR < 1.0 with liver herniation) randomized to either FETO (n = 21) at 26 to 30 weeks’ gestation or standard postnatal care (n = 20).45 The overall survival rate with severe CDH was significantly greater in the FETO intervention group than in the expectant management group (52.6% versus 5.3%). In 2009, a randomized Tracheal Occlusion To Accelerate Lung growth trial (TOTAL) was started.46 It compared postnatal management to late (30 to 32 weeks’ gestation) FETO intervention for moderate lung hypoplasia and earlier FETO intervention (27 to 30 weeks’ gestation) for severe lung hypoplasia. In addition, it is now understood that LHR depends on gestational age47 and that a ratio of observed to expected LHR is a better expression of CDH severity and likelihood of survival.48,49 This ratio is used as part of the ongoing TOTAL trial. Results of this trial will help determine if and when FETO should be offered for cases of severe CDH.
Congenital Pulmonary Airway Malformation
Congenital pulmonary airway malformations (CPAM) are pulmonary tumors with cystic and solid components; these malformations were previously described as congenital cystic adenomatoid malformations (CCAM).50 The incidence is approximately 1 in 25,000 pregnancies.51 The classification scheme for CPAM includes five subtypes, based on cyst size, characteristics of the epithelial lining, cyst wall thickness, and the presence of mucous cells, cartilage, and skeletal muscle.50,52 Lesions are assessed by ultrasonography and described as containing cysts larger (macrocystic) and smaller (microcystic) than 5 mm in diameter. Lesions can either regress with minimal associated morbidity or progressively enlarge, often resulting in hydrops fetalis (fetal heart failure). Small lesions detected in utero or in the newborn are treated after birth by surgical excision of the affected pulmonary lobe. Large lesions can cause mediastinal shift, hydrops, and pulmonary hypoplasia and can interfere with fetal or neonatal survival; fetuses with untreated lesions associated with hydrops fetalis have a survival rate of less than 5%.53 In a retrospective single-institution review of 71 cases, the initial antenatal ultrasonographic ratio of CPAM volume to fetal head circumference (CVR) was evaluated for the formation of hydrops fetalis and postnatal outcomes.54 Fetuses with a CVR less than 0.56 were noted to have no adverse postnatal outcomes, whereas a CVR greater than 0.56 had a positive predictive value for adverse postnatal outcome of 33%. In addition, a CVR greater than 1.6 was associated with a greater risk for hydrops fetalis.55
Depending on lesion size, location, and characteristics, CPAMs can be managed with either fetal intervention or postnatal resection. Macrocystic lesions have been decompressed in utero by placement of shunt catheters between the cysts and the amniotic cavity, resulting in sustained decompression and resolution of hydrops56; these procedures are followed by postnatal surgery. However, not all lesions can be decompressed successfully because the cysts are not always contiguous (i.e., in communication with each other) and can refill rapidly. In addition, these thoracoamniotic shunts have associated risks, including malfunction, displacement, fetal hemorrhage, and chorioamnionitis.57 CPAMs inappropriate for drainage can be resected with open fetal surgery. Fetal pulmonary lobectomy for lesions associated with hydrops fetalis has resulted in a 30-day postnatal survival rate of 50%, with tumor resection allowing for compensatory lung growth and resolution of hydrops fetalis.58 Maternal administration of betamethasone also has been noted to improve hydrops fetalis and overall outcome in selected fetuses with CPAM.59,60 A retrospective review of 24 fetuses with predominantly microcystic CPAM and the presence of hydrops fetalis found that corticosteroid treatment resulted in better survival than resection with open fetal surgery.61
Intralobar and extralobar pulmonary sequestrations (bronchopulmonary sequestrations) are rarer congenital lung anomalies than CPAM and involve nonfunctional lung tissue (disconnected from the bronchial tree). As with CPAM, therapeutic options depend on fetal morbidities including hydrops fetalis and pulmonary hypoplasia. Thoracoamniotic shunts have been successfully placed to decompress massive congenital pleural effusions caused by fetal chylothorax that otherwise result in hydrops fetalis, pulmonary compression, and fetal or neonatal death.62
Sacrococcygeal Teratoma
The prevalence of sacrococcygeal teratoma (SCT) is approximately 1 in 20,000 to 40,000.63 Some fetuses with SCT undergo massive tumor enlargement, experience hydrops fetalis and placentomegaly, and die in utero. These tumors function as large arteriovenous fistulas, and fetal demise results from high-output cardiac failure. Management of these tumors requires close surveillance because they can grow rapidly and reach a size as large as 1000 cubic centimeters.63 Fetuses with large lesions are at risk for intrapartum dystocia or tumor rupture and hemorrhage; these fetuses may require cesarean delivery. Fetuses with lesions diagnosed before 30 weeks’ gestation have a poor prognosis but may benefit from surgical debulking in utero; surgical techniques have not reached the necessary level of sophistication to allow complete resection of lesions that deeply invade the pelvis. In utero radiofrequency ablation and thermocoagulation have been used to reduce the tumor blood supply, but the benefit remains unclear.64 Catheterization of a fetal hand or umbilical cord vein for blood and crystalloid transfusion during tumor resection may be needed. To date, there has been no significant improvement in outcome with intervention in cases of SCT with hydropic fetalis.
Some SCT cases are accompanied by “maternal mirror syndrome” or Ballantyne syndrome, a hyperdynamic state (i.e., hypertension, peripheral and pulmonary edema) in which the maternal physiology mirrors the abnormal circulatory physiology of the hydropic fetus.65 This syndrome is associated with a substantial increase in fetal mortality and maternal morbidity and requires aggressive management similar to that used for severe preeclampsia, a disease from which it must be distinguished. Platelet count, aspartate aminotransferase, alanine aminotransferase, and haptoglobin are typically unaffected in maternal mirror syndrome and may serve as diagnostic clues to rule out severe preeclampsia and HELLP (hemolysis, elevated liver enzymes, low platelets). Unfortunately, maternal mirror syndrome typically does not resolve quickly, even with rapid correction of the fetal pathophysiology, and severe maternal complications including pulmonary edema occur in about 20% of cases.65
Myelomeningocele
Although not lethal, a myelomeningocele is a protrusion of the meninges and spinal cord through a congenital defect in the vertebrae and overlying muscles and skin. It can result in lifelong morbidity and disability, including paraplegia, bowel and bladder incontinence, hydrocephalus, Arnold-Chiari II malformation, and impaired cognition.66 Myelomeningocele has an incidence of about 1 in 2000 live births but is becoming less common owing to folate supplementation in the maternal diet. In addition, detection by ultrasonography and alpha-fetoprotein screening of maternal blood has allowed for earlier diagnosis (i.e., second trimester) and consideration of pregnancy termination.
The specific cause of myelomeningocele remains unknown. Animal models have demonstrated improved neonatal neurologic function with fetal closure of the defect in utero.67,68 The results associated with defect closure support a “two-hit” disease model in which the pathology is produced by failure of the fetal neural tube to form combined with prolonged exposure to the uterine amniotic fluid.69 Mutations of the PAX3 gene and direct cord trauma may also play a role in the pathophysiology associated with a myelomeningocele.70
The 5-year mortality of myelomeningocele is approximately 8% for live births; if it is not corrected in utero, surgical closure must be performed within a few days after birth.71 Ventriculoperitoneal shunting is required in 85% to 90% of uncorrected cases; however, despite successful shunting, permanent deficits such as central hypoventilation, vocal cord dysfunction, and oromotor and swallowing dysfunction can still occur from the associated Arnold-Chiari malformation.72 The average intelligence quotient in myelomeningocele patients who require ventriculoperitoneal shunting is 80 (low normal).73
The purpose of fetal surgery for myelomeningocele is to improve function later in life.69 Fetal surgery is primarily performed through an open fetal surgical technique. Preliminary results suggest that in utero repair successfully reverses the hindbrain herniation of the Arnold-Chiari II malformation, probably through normalization of cerebrospinal fluid flow, and decreases the need for ventriculoperitoneal shunt placement before 1 year of age.74 More recently, a randomized, prospective, multicenter clinical trial completed between 2003 and 2010 examined the risks and benefits of open fetal surgery for myelomeningocele repair compared with standard postnatal repair in 183 patients.75 Open fetal repair reduced the need for ventriculoperitoneal shunting and improved motor function at 30 months of age, but increased the risk for preterm birth and a partial or complete uterine dehiscence. Two perinatal deaths occurred in each group. Table 7-2 displays a subset of outcome measures that were significantly different between the prenatal and postnatal repair groups. Further data from the trial will include assessment of the long-term benefit from the prenatal intervention.
TABLE 7-2
Maternal and Fetal or Neonatal Complications for MOMS Trial Patients*
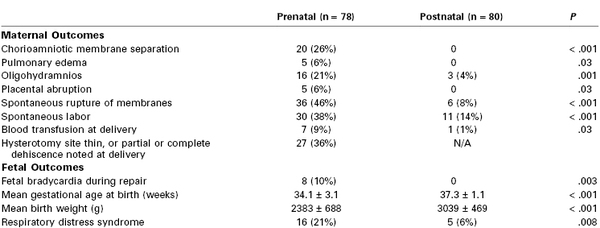
* The table lists maternal and fetal/neonatal complications that were significantly different (P < .05) between the prenatal and postnatal repair groups in the Management of Myelomeningocele Study (MOMS). Other outcomes were evaluated, but only those that were different between the two groups are included. Data for each group are shown as both an absolute number and as a percentage.
Modified from Adzick NS, Thom EA, Spong CY, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med 2011; 364:993-1004.
A recent study of endoscopic intrauterine myelomeningocele repair resulted in an extraordinarily high complication rate for both mothers and fetuses.76 Of the 19 study patients, three fetuses died intraoperatively and another three procedures were stopped owing to severe hemorrhage from the procedure. Although this intervention was associated with spinal segmental neuroprotection, it resulted in significantly more complications; the authors concluded that, pending further advances, this technique is unsuitable as standard care.76
Twin-to-Twin Transfusion Syndrome
An abnormal connection of chorionic blood vessels in the placenta between two monochorionic twins can result in twin-to-twin transfusion syndrome (TTTS). TTTS complicates 10% to 15% of monochorionic pregnancies, usually manifests at 15 to 26 weeks’ gestation, and is typically recognized at 20 to 21 weeks’ gestation.77,78 Intertwin transfusion is common between monochorionic twins and is usually balanced by the presence of arterioarterial (AA) and venovenous (VV) connections; the presence of AA connections is associated with a ninefold reduction in TTTS.79 By contrast, unidirectional and imbalanced blood flow through arteriovenous (AV) chorionic vessels results in TTTS. In normal fetoplacental vasculature, the umbilical artery branches at the placenta surface and traverses and then descends into the tissue, where it further branches into capillary divisions for gas and nutrient exchange. The arterial system is “paired” with venous vasculature, which returns blood to the umbilical cord. In TTTS, the umbilical artery similarly descends into the placenta and cotyledon, but rather than connecting with a paired vein it connects with a vein that transports blood to the other twin.80
The twin serving as the recipient demonstrates polycythemia, polyuria, polyhydramnios, and hypertrophic cardiomyopathy; this twin is at risk for hydrops fetalis and fetal death. The twin serving as the donor is typically hypovolemic, growth restricted, and pressed against the endometrium in an oligohydramniotic sac (hence the designation “stuck” or “pump” twin) and often has a velamentous cord insertion; this twin is at risk for neonatal renal failure, tubular dysgenesis and dysfunction, and high cardiac output hydrops fetalis. Diagnosis is currently based on ultrasonographic findings that focus on differences in fetal size or amniotic sac fluid volume, presence of cardiac dysfunction in the recipient twin, umbilical cord size, and abnormal umbilical arterial flow velocity.77,81 Twin size discordance is not always present and is no longer considered necessary to confirm the diagnosis. For unclear reasons, fetuses with TTTS are at risk for neurologic injury with white-matter lesions and long term disability; poor neurodevelopmental outcomes are associated with increased TTTS severity, delayed therapeutic interventions, and preterm delivery.82 If TTTS is untreated, it carries a greater than 80% mortality with 15% to 50% risk for significant morbidity in surviving neonates.83
A variety of therapeutic management techniques have been developed, including (1) serial amnioreduction to control polyhydramnios and reduce the risk for preterm labor, (2) surgical septostomy to equalize amniotic pressures, (3) selective feticide to allow the other fetus to survive, and (4) selective fetoscopic laser photocoagulation (SFLP) of the vascular anastomoses between the two twins. Serial amnioreduction was demonstrated to improve placental perfusion and decrease the occurrence of preterm delivery.84 In a retrospective review of 223 twin sets with TTTS, amnioreduction resulted in an overall birth survival rate of 78%, with 65% of recipient twins and 55% of donor twins alive at 1 month of age.85 In a prospective randomized trial comparing serial amnioreduction to septostomy, there was no difference in the rate of survival between the two techniques.86 Septostomy is rarely used for treatment because the creation of a single amniotic sac can increase the risk for umbilical cord entanglement.
The laser used for SFLP is typically inserted percutaneously through an endoscope (≤ 2.0 mm diameter) into the recipient twin’s amniotic sac. Maternal anesthesia is commonly managed with either neuraxial blockade or local anesthetic infiltration from skin to myometrium. Fetoscope placement is determined by placental location and is guided by ultrasonography. Vessels that cross the dividing membrane separating the amniotic sacs are visualized, and abnormal connecting vessels are selectively coagulated with the laser.77,80 On occasion, based on anatomic constraints created by the location of the fetuses and placenta, nonselective laser ablation is performed; however, this type of ablation is associated with higher rates of intrauterine fetal demise.77,87 Ablation of all abnormal connecting vasculature is not needed for success of the procedure.88 After completion of the SFLP, amniotic fluid may be removed to reduce the degree of polyhydramnios and possibly decrease the risk for preterm labor.
A 2004 randomized multicenter trial compared laser therapy to amnioreduction for treatment of severe TTTS diagnosed between 15 and 26 weeks’ gestation.89 Rates of at least one twin survival were significantly higher in the laser treatment group at both 28 days (76% versus 56%, P < .01) and 6 months of life (76% versus 51%, P < .01). In addition, neurologic outcomes were better in the laser treatment group. A subsequent prospective study of a large subgroup of survivors from this trial observed these children for 6 years and found no additional change in survival or long-term neurologic outcome from the original 6-month data.90 A meta-analysis of studies published between 1997 and 2007 noted that treatment of TTTS with laser ablation resulted in a higher overall rate of fetal survival than amnioreduction91; similar findings were demonstrated in a Cochrane review of treatment for TTTS.92
A more recent variation of the SFLP technique requires vascular laser ablation in a specific sequence. First, ablation occurs at the donor-to-recipient AV anastomoses, then at the recipient- to-donor AV anastomoses, then at the AA superficial anastomoses, and finally at the VV superficial anastomoses. The order of the procedure is designed to reduce the chance of hemodynamic compromise and hypotension during the procedure in the donor twin93; however, it is associated with longer operative times and increased case difficulty, particularly with an anterior placenta. In a single-institution study of consecutive SFLP for treatment of TTTS, twins treated with this sequence had better survival rates than twins whose SFLP procedure was performed without a specific sequence.93 A prospective multicenter trial found a significantly improved 30-day survival of both fetuses and improved donor survival with this sequential technique when compared with a cohort control group.94
The most common complication of SFLP is preterm PROM with subsequent preterm labor and delivery. Other possible complications include placement of the trocar through the placenta, hemorrhage, and possible membrane perforation resulting in limb entrapment and ischemia.77 In conclusion, trial results and meta-analyses provide evidence that SFLP results in superior outcomes than amnioreduction for the treatment of TTTS. Further research is needed to determine optimal techniques and timing of interventions for the treatment of TTTS.
Twin Reversed Arterial Perfusion Sequence
In monozygotic twins, one twin can also perfuse the other by retrograde blood flow though AA anastomoses. Twin reversed arterial perfusion (TRAP) sequence affects 1% of monozygotic twins and 1 in 30 triplets. Inadequate perfusion of the recipient twin via retrograde flow results in the development of a lethal set of anomalies that include acardia and acephalus. The normal (“pump”) twin perfuses both itself and the nonviable twin and is at risk for high-output congestive heart failure, polyhydramnios, and preterm birth. If untreated, TRAP sequence is associated with a risk for intrauterine death of the pump twin exceeding 50%.95 Diagnosis is confirmed with ultrasonographic demonstration of reverse flow to the acardiac twin via the umbilical artery. Cardiovascular failure in the pump twin is the indication for intervention, and early diagnosis is beneficial for optimal treatment.
The goal of therapy is to interrupt the vascular communication between the two twins. In contrast to the treatment of TTTS, treatment of TRAP sequence results in the death of the anomalous nonviable fetus. Percutaneous endoscopic laser or radiofrequency coagulation of the umbilical cord and/or placental vascular anastomoses is the most viable therapeutic option.96,97 Alternative therapies include sectio parva (selective cesarean delivery of one of multiple fetuses), percutaneous thrombosis of the acardiac twin’s umbilical cord with coils or other thrombogenic material, and alcohol-impregnated suture cord ligation. A retrospective review of 60 TRAP sequence cases from multiple European centers using endoscopic laser coagulation noted overall survival rates approaching 80% and a median gestational age of 37.4 weeks at delivery.98 An additional study, using radiofrequency coagulation in 26 TRAP sequence cases at a single U.S. medical center, demonstrated a 92% survival rate of the viable twin with a mean gestational age of 35.6 weeks at delivery.96 Both procedures are typically performed with infiltration of local anesthesia at the insertion site of the ablation device, although neuraxial anesthesia has also been used. The procedures are guided by ultrasonography, and absence of flow to the nonviable acardiac twin is confirmed with Doppler imaging at the end of the procedure and again 12 to 24 hours later.
Congenital Heart Defects
The most commonly performed closed fetal cardiac intervention for a congenital heart defect is an aortic valvuloplasty for mid-gestational aortic stenosis with evolving hypoplastic left heart syndrome. Technical success as high as 75% has been reported using an angioplasty balloon over a guidewire inserted percutaneously through an access cannula.99 Approximately 40% of successful cases result in aortic regurgitation and minimal subsequent left ventricular growth; however, the physiology of the left ventricle improves and leads to improved aortic and mitral valvular growth. In about 30% of the successful cases, biventricular circulation is present at birth. Other congenital heart defects that may benefit from antepartum closed fetal cardiac intervention include (1) hypoplastic left heart syndrome with an intact or highly restrictive atrial septum and (2) evolving hypoplastic right heart syndrome with pulmonary atresia or stenosis without a ventricular septal defect.100,101
More complex congenital cardiac defects that might benefit from open repair have only been repaired in animal models and require fetal extracorporeal circulatory bypass; the use of fetal cardiac bypass induces a catecholamine-mediated fetal stress response that increases vascular resistance and cardiac afterload and is poorly tolerated by the immature fetal myocardium. Fetal cardiac bypass can also result in severe placental dysfunction when the high-capacitance, low-resistance placenta is incorporated as the oxygenator, resulting in endothelial dysfunction and leukocyte and complement activation. After fetal cardiac bypass, increases in placental vascular resistance, reduced blood flow, impaired gas exchange, and fetal acidosis are frequently observed.102,103 Correction of complex congenital cardiac defects, either open or closed, requires careful anesthetic and pharmacologic strategies for myocardial protection.
Surgical Benefits and Risks
The primary goal of intrauterine fetal surgery is to improve neonatal outcomes over that of surgery performed after a preterm or term delivery. The intrauterine environment supports rapid wound healing (i.e., without scarring before mid gestation),104 and the umbilical circulation meets nutritional and respiratory needs without outside assistance. The poorly developed fetal immune surveillance system may facilitate certain invasive procedures. However, continued refinement of surgical and anesthetic techniques, reduction of maternal and fetal risk, and appropriate clinical trials for each intervention must occur before fetal surgery can be performed on a more routine basis for a given congenital anomaly.
Serious maternal complications from intrauterine fetal surgery are relatively uncommon. Maternal risks include blood loss, infection, placental abruption, and pulmonary edema secondary to tocolytic therapy and fluid overload from absorption of significant amounts of pressurized crystalloid uterine irrigation during fetoscopic techniques.105,106 Open fetal surgery involves a hysterotomy that is not in the lower uterine segment, and therefore all future deliveries must occur via a cesarean procedure. Maternal welfare must always be emphasized.7
The fetal risks of intrauterine surgery remain relatively high. The most common postoperative complications are fetal central nervous system injuries, postoperative amniotic fluid leaks, membrane separation, preterm PROM, and preterm labor and delivery. Preterm delivery accounts for significant morbidity and mortality among fetuses that might otherwise have benefited from the therapeutic interventions. Chorioamniotic membrane separation can cause amniotic bands, umbilical cord strangulation, and fetal demise.107
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree