Background
The integrity of the pelvic autonomic nervous system is essential for proper bowel, bladder, and sexual function.
Objective
The purpose of this study was to characterize the anatomic path of the pelvic autonomic system and to examine relationships to clinically useful landmarks.
Study Design
Detailed dissections were performed in 17 female cadavers. Relationships of the superior hypogastric plexus to aortic bifurcation and midpoint of sacral promontory were examined; the length and width of plexus was documented. Path and width of right and left hypogastric nerves were recorded. The origin and course of the pelvic splanchnic nerves were documented. Individual nerve tissue that contributed to the inferior hypogastric plexus was noted. Relative position of nerves to arteries, viscera, and ligaments was documented. In a subset of specimens, biopsy specimens were obtained to confirm gross findings by histologic analysis. Descriptive statistics were used for data analyses and reporting.
Results
In all specimens, the superior hypogastric plexus was embedded in a connective tissue sheet within the presacral space, just below the peritoneum. In 14 of 17 specimens (82.4%), the plexus formed a median distance of 21.3 mm (range, 9–40 mm) below aortic bifurcation; in the remaining specimens, it formed a median distance of 25.3 mm (range, 20.5–30 mm) above bifurcation. In 58.8% of specimens, the superior hypogastric plexus was positioned to the left of midline. The median length and width of the plexus was 39.5 (range, 11.5–68) mm and 9 (range, 2.5–15) mm, respectively. A right and left hypogastric nerve was identified in all specimens and formed a median distance of 23 mm (range, 5–32 mm) below the promontory. The median width of the hypogastric nerve was 3.5 mm (range, 3–4.5 mm) on the right and 3.5 mm (range, 2–6.5 mm) on the left. The median distance from midportion of uterosacral ligament to the closest nerve branch was 0.5 mm (range, 0-4.5 mm) on right and 0 mm (range, 0-27.5 mm) on left. In all specimens, the inferior hypogastric plexus was formed by contributions from the hypogastric nerves and branches from S3 and S4. In 47.1% of hemipelvises, S2 branches contributed to the plexus. The sacral sympathetic trunk contributed to the plexus in 16 of 34 hemipelvises where this structure was identified. The inferior hypogastric plexus formed 1–3 cm lateral to the rectum and upper third of the vagina. From this plexus, 1–3 discrete branches coursed deep to the ureter toward the bladder. A uterine branch that coursed superficial to the ureter followed the ascending branch of the uterine artery. An S4 branch was found directly attaching to lateral walls of the rectum in 53% of specimens. Pelvic splanchnic nerves merged into the inferior hypogastric plexus on the lower and medial surface of the coccygeus muscle. Histologic analysis confirmed neural tissue in all tissues that were sampled.
Conclusion
Anatomic variability and inability to visualize the small caliber fibers that comprise the inferior hypogastric plexus grossly likely underlines the reasons that some postoperative visceral and sexual dysfunction occur in spite of careful dissection and adequate surgical technique. These findings highlight the importance of a discussion with patients about the risks that are associated with interrupting autonomic fibers during the preoperative consent.
Integrity of the pelvic autonomic nervous system is essential for proper bowel, bladder, and sexual function. Radical pelvic surgeries that remove portions of this system lead to significant visceral and/or sexual dysfunction. For example, radical hysterectomy is associated with 70–85% rates of bladder dysfunction, 17–91% defecatory dysfunction, and 33–65% sexual dysfunction. In addition, procedures that intentionally interrupt the pelvic autonomic system to treat refractory pelvic pain, such as presacral neurectomy and uterosacral nerve ablation, are also associated with visceral and sexual dysfunction.
The pelvic autonomic system is composed of the superior and inferior hypogastric plexuses. The superior hypogastric plexus (SHP), often called the presacral nerve, represents the distal extension of the aortic and inferior mesenteric plexuses and terminates by dividing into right and left hypogastric nerves. The SHP consists primarily of sympathetic and visceral afferent fibers. The inferior hypogastric plexus (IHP) is found in the deep extraperitoneal spaces adjacent to the pelvic viscera and is formed by contributions from the hypogastric nerves, pelvic splanchnic nerves, and sacral sympathetic chain or trunk. Accordingly, the IHP is a mixed plexus that consists of both sympathetic and parasympathetic fibers that are distributed to all pelvic viscera and to the erectile structures in the perineum.
Impaired quality of life in cancer survival patients has led to many technical descriptions for nerve-sparing radical surgeries. Of these, radical hysterectomy, prostatectomy, and rectal excision have received the most attention. However, less emphasis to nerve-sparing dissection is placed during hysterectomy for benign conditions or pelvic reconstructive surgery. Yet, these procedures often involve dissection in the deep pelvic retroperitoneal spaces and potential exists for autonomic disruption. Indeed, de novo visceral dysfunction has been reported after apical reconstructive procedures. Furthermore, visceral and sexual dysfunction has been reported after hysterectomy. Although the precise cause is unknown, new onset organ dysfunction after these procedures may be related to disruption of the pelvic autonomic system.
The objectives of this study were to characterize further the path and relationships of the individual components of the pelvic autonomic nerve plexuses and to explore the anatomic rationale for visceral and sexual dysfunction that occasionally is seen after pelvic surgery.
Materials and Methods
Detailed dissections were performed on 17 female cadavers that were obtained from the Willed Body Program at the University of Texas Southwestern in Dallas, TX. This study was exempt from review by the University of Texas Southwestern Institutional Review Board in accordance with the Code of Federal Regulations, Title 45. Age, race, height, weight, and cause of death were obtained for all specimens.
After 3 practice dissections (not included in data analyses) that were supervised by 2 of the authors (J.N.P. and M.M.C.), all contributing authors participated in the exposure of the SHP and the proximal segment of the hypogastric nerves. The distal extent of the hypogastric nerves and IHP were dissected by the urogynecology fellow (C.M.R.) and faculty (M.M.C.). Practice dissections were performed in efforts to ensure that all authors understood the anatomic structures and relationships to be examined and agreed on the measurements that would be taken.
Cadavers were transected superior to the origin of the inferior mesenteric artery and at the midportion of the thighs. The sigmoid colon was tied above the pelvic brim; before any dissection, the uterosacral ligament (USL) was palpated, and a suture was placed through the midportion (level of ischial spines) of each ligament. The peritoneum overlying the aortic bifurcation and the sacrum was carefully dissected to expose the SHP and the proximal portions of the hypogastric nerves. The origin, appearance (plexiform vs single nerve), position relative to the midline, length, and width of the SHP were recorded. The relationship of the SHP to the aortic bifurcation and to the midpoint of the sacral promontory was also documented. As described by Weislander et al, the location of the midpoint of the sacral promontory was determined as the midpoint between the junctions of the body of the anterior surface of the first sacral vertebra (S1) with the ala of the sacrum. The distance from midpoint of the sacral promontory to the origin of the hypogastric nerves and the nerve width at its origin were measured. The sacral sympathetic trunk (SST) was identified at the level of the pelvic inlet, and its transverse distance to the midpoint of the sacral promontory was recorded.
The SHP was then cut in the midline, and each side was tagged with a suture for later identification. Cadavers were then transected in the midsagittal plane, and detailed dissections were carried out on each hemipelvis. The course of the hypogastric nerves was followed; their relationships to the rectum, uterus, and USL were documented. The fascia covering the piriformis muscles was carefully dissected to expose the ventral rami of the first through fourth sacral nerves (S1-S4) and the SST. Visceral branches arising from S2-S4 (pelvic splanchnic nerves) were exposed carefully, and their specific origin was identified. The course, termination, and position of the pelvic splanchnic nerves relative to the pelvic viscera, coccygeus-sacrospinous ligament (C-SSL) complex, and sacrum were annotated. Branches from sacral nerves to the coccygeus muscles were also identified, and their origins were recorded. The course of the uterine, vaginal, and middle rectal arteries was followed; their positions relative to the pelvic splanchnic nerves or other components of the IHP was recorded. When a middle rectal artery was identified, its origin and width were noted. The path of the SST was followed, and its contribution to the IHP, when noted, was documented. The connective tissue bundle where the hypogastric nerves and the pelvic splanchnic nerves converged was identified and labeled IHP. The morphologic variations and position of the IHP relative to the pelvic viscera, ligaments, and vessels was annotated. At this point, dissection was continued with the use of ×2.5 magnification lenses to facilitate identification of individual nerve branches to the bladder, uterus, vagina, and rectum.
All measurements were taken twice by the same examiner (C.M.R.) who used the same steel electrocardiogram caliper and plastic ruler. The mean of the 2 measurements was used for analyses. Measurements were tabulated; descriptive statistics were used for data analysis and reporting with the use of Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA). In a subset of not embalmed specimens, biopsy specimens were obtained to confirm gross findings by histologic examination. Tissue harvested for microscopic analysis included the SHP, components of the IHP, and the USL. Tissue was fixed in formalin, embedded in paraffin, and stained with hematoxylin and eosin. Tissue sections were examined for the presence of neural tissue by a faculty pathologist (K.S.C.) on a Nikon Eclipse 80i microscope (Nikon Instruments Inc, Melville, NY).
Results
A total of 17 (13 not embalmed and 4 embalmed) cadavers or 34 hemipelvises were examined. All cadavers were white with a median age of 80 years (range, 63–97 years) and body mass index of 19.2 kg/m 2 (range, 10.0–30.5 kg/m 2 ). Four specimens (23.5%) had a previous hysterectomy. Dissections and available medical histories revealed no obvious signs of pelvic disease, such as cancer or previous pelvic fractures. None of the specimens had prolapse that extended beyond the hymen when traction was applied to the cervix. Parity of the specimens was not known. Meaningful autonomic nerve findings relative to surgical landmarks are outlined in the Table .
| Nerve structure | Measurement, median (range) |
|---|---|
| Superior hypogastric plexus, mm | |
| Distance from origin to aortic bifurcation | |
| Below (n=14) | 21.3 (9–40) |
| Above (n=3) | 25.3 (20.5–30) |
| Length | 39.5 (11.5–68) |
| Width | 9 (2.5–15) |
| Hypogastric nerves, mm | |
| Distance from origin to midpoint of sacral promontory | 23 (5–32) |
| Width | |
| Right | 3.5 (3–4.5) |
| Left | 3.5 (2–6.5) |
| Inferior hypogastric plexus, mm | |
| Distance from closest inferior hypogastric plexus nerve to midportion of uterosacral ligament | |
| Right | 0.5 (0–4.5) |
| Left | 0 (0–27.5) |
SHP
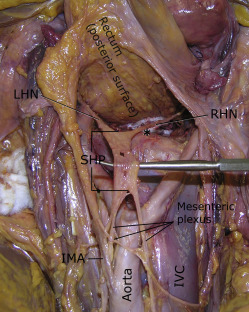
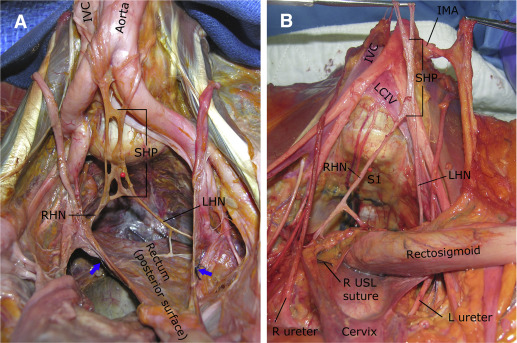
In all specimens, the SHP was embedded in a sheet of fatty connective tissue within the presacral space, just below the peritoneum. In 14 of 17 specimens (82.4%), the SHP formed below the aortic bifurcation; in the remaining 3 specimens (17.6%), the SHP formed above the bifurcation. The SHP formed from extensions of the mesenteric plexus, and the nerve contributions coursed parallel to the aorta as single or multiple fibers ( Figure 1 ). On the right, contributing fibers always were noted between the aorta and vena cava; on the left, nerve fibers coursed to the left of the aorta, and most were densely attached to the undersurface of the inferior mesenteric artery. The SHP formed to the left of the midline in 58.8% of specimens and in the midline in 41.2%. In 16 of 17 specimens (94%), the SHP had a fenestrated or plexiform appearance; in 1 of 17 specimens, a single cord-like structure was noted ( Figure 2 ).
Hypogastric nerves
A right and left hypogastric nerve was identified in all specimens. In 17.6% of specimens, 2 hypogastric nerves were noted on the right; in 11.8%, 2 nerves were noted on the left. In all specimens, the hypogastric nerves formed below the sacral promontory. These nerves coursed inferiorly and laterally within the presacral space toward the sides of the upper rectum ( Figures 1 and 2 ). Communicating fibers between the right and left hypogastric nerves were often found, but none were noted to penetrate the posterior wall of the rectum.
IHP
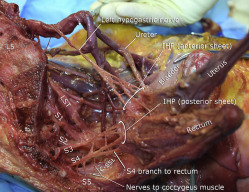
In all specimens, the IHP was formed by contributions from the SHP via the hypogastric nerves and by visceral branches from the ventral rami of the third and fourth sacral nerves (S3 and S4; Figure 3 ). A contribution from S2 was noted in 47.1% of specimens: 52.9% in the right and 41.2% in the left hemipelvises. The SST coursed medial or just anterior to the sacral foramina. This trunk was easiest to identify at the first sacral vertebral level but was generally attenuated at lower sacral levels, which made it difficult to follow its path accurately to determine its contributions to the IHP in 18 of 34 hemipelvises. However, in all 16 hemipelvises in which this structure was identified accurately distally, contributions to the IHP were noted ( Figure 4 ). In these cases, branches from the SST ganglia to the ventral rami of each sacral spinal nerve were also noted.
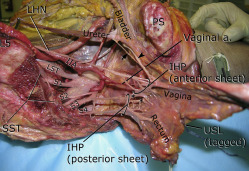
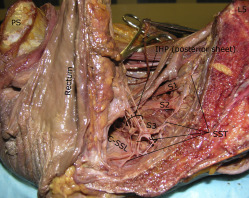
Approximately at the level of the midportion of the USLs, the distal portion of the hypogastric nerves consistently “fanned out” into a triangular sheet of tissue that contained many thin (≤2 mm) nerve fibers that were embedded in loose connective tissue. Visceral branches from S2–S4 (pelvic splanchnic nerves) coursed toward the lateral border of the upper rectum and became entangled into another plexiform sheet-like structure at a more posterior level than the hypogastric nerve sheet ( Figure 5 ). This posterior sheet of nerve tissue communicated with the anterior sheet lateral and inferior to the USL’s midpoint, between the vagina and rectum ( Figures 3 and 5 ). Direct branches from S4 entered the lateral wall of the rectum in 53% of specimens ( Figure 5 ). The anterior sheet of the IHP consistently formed 1–3 cm lateral to the upper third of the posterior vagina. The posterior sheet formed 1–3 cm lateral to the rectum and was identified on the medial and lower border of the C-SSL complex.