General Considerations
Asthma is the most common chronic disease of childhood, affecting over 7 million children in the United States. While current prevalence rates for asthma have increased in the past decade (most recent estimate of 10%), the rate of asthma attack in the past year has been stable. Gender, race, and socioeconomic disparities in the prevalence of asthma exist: (1) More boys than girls are affected in childhood; (2) Higher percentage affected among black children compared to Hispanic and non-Hispanic white children; (3) Children belonging to poor families are more likely to be affected.
There is still a disproportionately higher healthcare utilization for asthma among children compared to adults affected by this disease. Asthma health care encounters in primary care settings have increased over time; death rates and emergency department (ED) visits related to asthma have declined, and hospitalizations due to asthma have been steady. Hospitalizations and emergency department or urgent ambulatory or office visits, all indicators of asthma severity, impose significant costs to the healthcare system and to families, caretakers, schools, and parents’ employers. Indirect costs primarily from loss of productivity due to school/work absences are harder to measure, yet considerable. Asthma remains a potentially life-threatening disease for children; the rate of asthma deaths was 28 per 1 million children with current asthma. Similar to disparities in prevalence, morbidity and mortality rates for asthma are higher among minority and inner city populations. The reasons for this are unclear but may be related to a combination of more severe disease, poor access to health care, lack of asthma education, delay in use of appropriate controller therapy, and environmental factors (eg, irritants including smoke and air pollutants, and perennial allergen exposure).
Up to 80% of children with asthma develop symptoms before their fifth birthday. Atopy (personal or familial) is the strongest identifiable predisposing factor. Sensitization to inhalant allergens increases over time and is found in the majority of children with asthma. The principal allergens associated with asthma are perennial aeroallergens such as dust mite, animal dander, cockroach, and Alternaria (a soil mold). Rarely, foods may provoke isolated asthma symptoms.
About 40% of infants and young children who have wheezing with viral infections in the first few years of life will have continuing asthma through childhood. Viral infections (eg, respiratory syncytial virus [RSV], rhinovirus, parainfluenza and influenza viruses, metapneumovirus) are associated with wheezing episodes in young children. RSV may be the predominant pathogen of wheezing infants in the emergency room setting, but rhinovirus can be detected in the majority of older wheezing children. Furthermore, RSV and parainfluenza have been associated with more severe respiratory illnesses, but in general, rhinovirus is the most commonly identified respiratory virus with wheezing episodes. It is uncertain if these viruses contribute to the development of chronic asthma, independent of atopy. Severe RSV bronchiolitis in infancy has been linked to asthma and allergy in childhood. Although speculative, individuals with lower airways vulnerability to common respiratory viral pathogens may be at risk for persistent asthma.
In addition to atopy and infections being associated with the development of asthma, observational studies have also demonstrated an increased risk of asthma attributed to acetaminophen exposure during prenatal periods, infancy, childhood, and even adulthood. Acetaminophen is the most commonly used antipyretic medication for children in the United States. Furthermore, there is evidence from secondary analyses suggesting that acetaminophen exposure increases the risk for subsequent asthma exacerbations or wheeze compared to ibuprofen; and that a dose dependent elevated risk of asthma symptoms could be found.
There are several mechanisms which have been proposed: acetaminophen interfering with glutathione (a tripeptide antioxidant that is involved in free radical scavenging and xenobiotic detoxification) pathway and impairing respiratory antioxidant defenses; presence of genetic polymorphisms in the glutathione pathway that are associated with increased susceptibility to asthma; and acetaminophen causing a switch to a TH2 from a TH1 response. Stronger pieces of evidence such as prospectively designed studies primarily addressing the questions of whether acetaminophen exposure truly increases the risk of the development of chronic asthma or even triggers acute asthma are needed.
Exposure to tobacco smoke, especially from the mother, is also a risk factor for asthma. Other triggers include exercise, cold air, cigarette smoke, pollutants, strong chemical odors, and rapid changes in barometric pressure. Aspirin sensitivity is uncommon in children. Psychological factors may precipitate asthma exacerbations and place the patient at high risk from the disease.
Pathologic features of asthma include shedding of airway epithelium, edema, mucus plug formation, mast cell activation, and collagen deposition beneath the basement membrane. The inflammatory cell infiltrate includes eosinophils, lymphocytes, and neutrophils, especially in fatal asthma exacerbations. Airway inflammation contributes to airway hyperresponsiveness, airflow limitation, and disease chronicity. Persistent airway inflammation can lead to airway wall remodeling and irreversible changes.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
The diagnosis of asthma in children, especially among preschool aged, is based largely on clinical judgment and an assessment of symptoms, activity limitation, and quality of life. For example, if a child with asthma refrains from participating in physical activities so as not to trigger asthma symptoms, their asthma would be inadequately controlled but not detected by the standard questions. In the National Asthma Education and Prevention Program (NAEPP) clinical guidelines, asthma control is introduced as an approach to assess the adequacy of current treatment, and to improve care and outcomes for children with asthma. For children with asthma, numerous validated instruments and questionnaires for assessing health-related quality of life and asthma control have been developed. The Asthma Control Test (ACT, www.asthmacontrol.com), the Asthma Control Questionnaire (ACQ, www.qoltech.co.uk/Asthma1.htm), and the Asthma Therapy Assessment Questionnaire (ATAQ, www.ataqinstrument.com) for children 12 years of age and older, and the Childhood ACT for children 4–11 years of age are examples of self-administered questionnaires that have been developed with the objective of addressing multiple aspects of asthma control such as frequency of daytime and nocturnal symptoms, use of reliever medications, functional status, missed school or work, and so on. A five-item caregiver-administered instrument, the Test for Respiratory and Asthma Control in Kids (TRACK), has been validated as a tool to assess both impairment and risk presented in the NAEPP Expert Panel Report 3 (EPR3) guidelines in young children with recurrent wheezing or respiratory symptoms consistent with asthma.
Wheezing is the most characteristic sign of asthma, although some children may have recurrent cough and shortness of breath. Complaints may include “chest congestion,” prolonged cough, exercise intolerance, dyspnea, and recurrent bronchitis or pneumonia. Chest auscultation during forced expiration may reveal prolongation of the expiratory phase and wheezing. As the obstruction becomes more severe, wheezes become more high-pitched and breath sounds diminished. With severe obstruction, wheezes may not be heard because of poor air movement. Flaring of nostrils, intercostal and suprasternal retractions, and use of accessory muscles of respiration are signs of severe obstruction. Cyanosis of the lips and nail beds may be seen with underlying hypoxia. Tachycardia and pulsus paradoxus also occur. Agitation and lethargy may be signs of impending respiratory failure.
B. Laboratory Findings
Bronchial hyperresponsiveness, reversible airflow limitation, and airway inflammation are key features of asthma. Documentation of all these components is not always necessary, unless the presentation is rather atypical.
Bronchial hyperresponsiveness to nonspecific stimuli is a hallmark of asthma. These stimuli include inhaled pharmacologic agents such as histamine, methacholine, and mannitol, as well as physical stimuli such as exercise and cold air. Mannitol (Aridol) bronchoprovocation has been approved by the FDA and is simpler and easier to administer in the office. It is available as a dry powder inhalation kit, and takes less time to complete. Unlike methacholine and histamine challenges and similar to exercise challenge, it is considered an indirect challenge, that is, it simulates airway responses to specific physiologic situations, by creating an osmotic effect within the airway that subsequently leads to an inflammatory response. Airways may exhibit hyperresponsiveness or twitchiness even when baseline pulmonary function tests are normal. Giving increasing concentrations of a bronchoconstrictive agent to induce a decrease in lung function (usually a 20% drop in forced expiratory volume in 1 second [FEV1] for histamine and methacholine and a 15% reduction for mannitol) and doing an exercise challenge are ways to determine airway responsiveness. Hyperresponsiveness in normal children younger than age 5 years is greater than in older children. The level of airway hyperresponsiveness usually correlates with the severity of asthma. Bronchoprovocation challenges may help to establish a diagnosis of asthma when the history, examination, and pulmonary function tests are not definitive.
Recent asthma clinical guidelines reinforce the use of spirometry over peak expiratory flow rate (PEFR) measurements, in the evaluation of airflow limitation in asthma. This can be measured by reduction in FEV1 and FEV1/FVC values compared to reference or predicted values. By itself, it is not adequate in establishing a diagnosis, but it can be an important parameter to monitor asthma activity and treatment response. In children, FEV1 may be normal, despite frequent symptoms. Spirometric measures of airflow limitation can be associated with symptom severity, likelihood of exacerbation, hospitalization, or respiratory compromise. Regular monitoring of prebronchodilator (and ideally postbronchodilator) FEV1 can be used to track lung growth patterns over time. During acute asthma exacerbations, FEV1 is diminished and the flow-volume curve shows a “scooping out” of the distal portion of the expiratory portion of the loop (Figure 38–1).
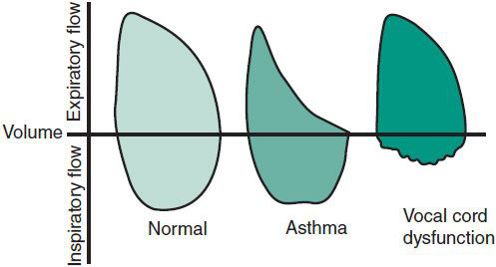
 Figure 38–1. Representative flow-volume loops in persons with normal lung function, asthma, and vocal cord dysfunction.
Figure 38–1. Representative flow-volume loops in persons with normal lung function, asthma, and vocal cord dysfunction.
Lung function assessment using body box plethysmography to determine lung volume measurements can also be informative. The residual volume, functional residual capacity, and total lung capacity are usually increased, while the vital capacity is decreased. Reversal or significant improvement of these abnormalities in response to inhaled bronchodilator therapy or with anti-inflammatory therapy can be observed.
PEFR monitoring can be a simple and reproducible tool to assess asthma activity in children with moderate or severe asthma, a history of severe exacerbations, or poor perception of airflow limitation or worsening condition. Significant changes in PEFR may occur before symptoms become evident. In more severe cases, PEFR monitoring enables earlier recognition of suboptimal asthma control.
Infant pulmonary function can be measured in sedated children with compression techniques. The forced oscillation technique can be used to measure airway resistance even in younger children.
Hypoxemia is present early with a normal or low Pco2 level and respiratory alkalosis. Hypoxemia may be aggravated during treatment with a β2-agonist due to ventilation-perfusion mismatch. Oxygen saturation less than 91% is indicative of significant obstruction. Respiratory acidosis and increasing CO2 tension may ensue with further airflow obstruction and signal impending respiratory failure. Hypercapnia is usually not seen until the FEV1 falls below 20% of predicted value. Metabolic acidosis has also been noted in combination with respiratory acidosis in children with severe asthma and indicates imminent respiratory failure. Pao2 less than 60 mm Hg despite oxygen therapy and Paco2 over 60 mm Hg and rising more than 5 mm Hg/h are relative indications for mechanical ventilation in a child in status asthmaticus.
Pulsus paradoxus may be present with moderate or severe asthma exacerbation. In moderate asthma exacerbation in a child, this may be between 10 and 25 mm Hg, and in severe asthma exacerbation between 20 and 40 mm Hg. Absence of pulsus paradoxus in a child with severe asthma exacerbation may signal respiratory muscle fatigue.
Clumps of eosinophils on sputum smear and blood eosinophilia are frequent findings. Their presence tends to reflect disease activity and does not necessarily mean that allergic factors are involved. Leukocytosis is common in acute severe asthma without evidence of bacterial infection and may be more pronounced after epinephrine administration. Hematocrit can be elevated with dehydration during prolonged exacerbations or in severe chronic disease. Noninvasive measures of airway inflammation include exhaled nitric oxide concentrations, serum eosinophil cationic protein levels, and induced sputum. Each test has its strengths and weaknesses.
C. Imaging
Evaluation of asthma usually does not need chest radiographs (posteroanterior and lateral views) since they often appear normal, although subtle and nonspecific findings of hyperinflation (flattening of the diaphragms), peribronchial thickening, prominence of the pulmonary arteries, and areas of patchy atelectasis may be present. Atelectasis may be misinterpreted as the infiltrates of pneumonia. Some lung abnormalities, such as bronchiectasis, which may point to a different diagnosis implicating an asthma masquerader, such as cystic fibrosis, allergic bronchopulmonary mycoses (aspergillosis), ciliary dyskinesias, or immune deficiencies, can be better appreciated with high-resolution, thin-section chest computed tomography (HRCT) scans. It is primarily useful clinically in ruling out certain diagnoses in patients with difficult to manage asthma but radiation exposure should be considered when ordering HRCT.
Allergy testing is discussed in the section on General Measures under Treatment, Chronic Asthma.
 Differential Diagnosis
Differential Diagnosis
Diseases that may be mistaken for asthma are often related to the patient’s age (Table 38–1). Congenital abnormalities must be excluded in infants and young children. Asthma can be confused with croup, acute bronchiolitis, pneumonia, and pertussis. Immunodeficiency may be associated with cough and wheezing. Foreign bodies in the airway may cause dyspnea or wheezing of sudden onset, and on auscultation, wheezing may be unilateral. Asymmetry of the lungs secondary to air trapping may be seen on a chest radiograph, especially with forced expiration. Cystic fibrosis can be associated with or mistaken for asthma.
Table 38–1. Differential diagnosis of asthma in infants and children.
Vocal cord dysfunction is an important masquerader of asthma, although the two can coexist. It is characterized by the paradoxic closure of the vocal cords that can result in dyspnea and wheezing. Diagnosis is made by direct visualization of the vocal cords. In normal individuals, the vocal cords abduct during inspiration and may adduct slightly during expiration. Asthmatic patients may have narrowing of the glottis during expiration as a physiologic adaptation to airway obstruction. In contrast, patients with isolated vocal cord dysfunction typically show adduction of the anterior two-thirds of their vocal cords during inspiration, with a small diamond-shaped aperture posteriorly. Because this abnormal vocal cord pattern may be intermittently present, a normal examination does not exclude the diagnosis. Bronchial challenges using exercise or methacholine can precipitate symptoms of vocal cord dysfunction. The flow-volume loop may provide additional clues to the diagnosis of vocal cord dysfunction. Truncation of the inspiratory portion can be demonstrated in most patients during an acute episode, and some patients continue to show this pattern even when they are asymptomatic (see Figure 38–1). Children with vocal cord dysfunction, especially adolescents, tend to be overly competitive, primarily in athletics and scholastics. A psychiatric consultation may help define underlying psychological issues and provide appropriate therapy. Treatment of isolated vocal cord dysfunction includes education regarding the condition and appropriate breathing exercises. Hypnosis, biofeedback, and psychotherapy have been effective for some patients.
 Conditions That May Increase Asthma Severity
Conditions That May Increase Asthma Severity
Chronic hyperplastic sinusitis is frequently found in association with asthma. Upper airway inflammation has been shown to contribute to the pathogenesis of asthma, and asthma may improve after treatment of sinusitis. However, sinus surgery is usually not indicated for initial treatment of chronic mucosal disease associated with allergy. In older children, rarely, hyperplastic sinusitis and polyposis and severe refractory asthma can be associated with aspirin sensitivity, known as aspirin-exacerbated respiratory disease (AERD).
A significant correlation has been observed between nocturnal asthma and gastroesophageal reflux. Patients may not complain of burning epigastric pain or have other reflux symptoms—cough may be the only sign. For patients with poorly controlled asthma, particularly with a nocturnal component, investigation for gastroesophageal reflux may be warranted even in the absence of suggestive symptoms.
Population studies have demonstrated associations between obesity and asthma. Obesity has been linked not only to the development of asthma but also with asthma control and severity. What contributes to these associations or to what extent inflammation or physiologic impairment relates to both obesity and asthma is less established. It becomes difficult to determine if a child’s trouble breathing is a result of obesity itself, its comorbidities (eg, gastroesophageal reflux or obstructive sleep apnea), and/or asthma. A management approach targeting weight reduction in obese children is encouraged to improve asthma control or its assessment.
The risk factors for death from asthma include psychological and sociological factors. They are probably related to the consequences of illness denial, poor coping or self-management skills, as well as to nonadherence with prescribed therapy. Recent studies have shown that less than 50% of inhaled asthma medications are taken as prescribed and that compliance does not improve with increasing severity of illness. Moreover, children requiring hospitalization for asthma, or their caregivers, have often failed to institute appropriate home treatment.
 Complications
Complications
With acute asthma, complications are primarily related to hypoxemia and acidosis and can include generalized seizures. Pneumomediastinum or pneumothorax can be a complication in status asthmaticus. With chronic asthma, recent studies point to airway wall remodeling and loss of pulmonary function with persistent airway inflammation. Childhood asthma independent of any corticosteroid therapy has been shown to be associated with delayed maturation and slowing of prepubertal growth velocity.
 Treatment
Treatment
A. Chronic Asthma
1. General measures—Optimal asthma management includes an assessment and regular monitoring of disease activity, education and partnership to improve the child’s and his/her family’s knowledge and skills for self-management, identification and management of triggers and conditions that may worsen asthma, and appropriate medications selected to address the patient’s needs. The objective of asthma management is the attainment of the best possible asthma control.
An assessment of asthma severity (ie, the intrinsic intensity of disease) is generally most accurate in patients not receiving controller therapy. Hence, assessing asthma severity directs the level of initial therapy. For those already on treatment, asthma severity can be classified according to the level of medication requirement to maintain adequate asthma control. The two general categories are intermittent and persistent asthma, the latter further subdivided into mild, moderate, and severe (Table 38–2). In contrast, asthma control refers to the degree to which symptoms, ongoing functional impairments, and risk of adverse events are minimized and goals of therapy are met. Assessment of asthma control should be done at every visit as this is important in adjusting therapy. It is categorized as “well controlled,” “not well controlled,” and “very poorly controlled” (Table 38–3). Responsiveness to therapy is the ease with which asthma control is attained by treatment. It can also encompass monitoring for adverse effects related to medication use.
Table 38–2. Assessing severity and initiating treatment for patients who are not currently taking long-term control medications.
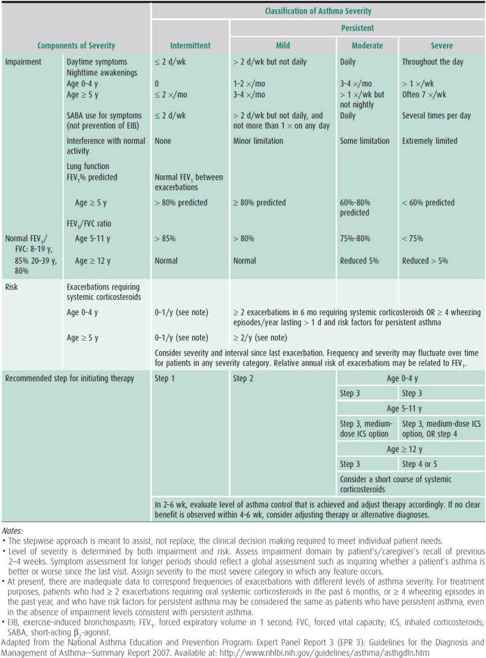
Table 38–3. Assessing asthma control and adjusting therapy in children.
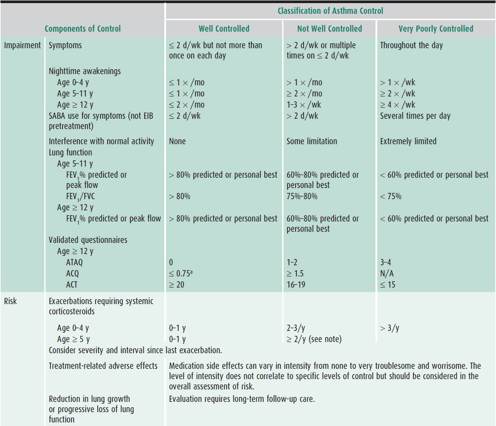
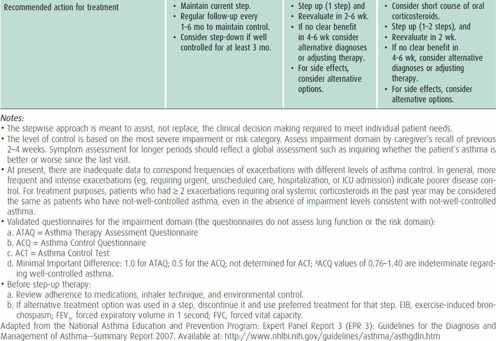
Classification of either asthma severity or control is based on the domains of current impairment and risk, recognizing that these domains may respond differently to treatment. The level of asthma severity or control is established upon the most severe component of impairment or risk. Generally, the assessment of impairment is symptom based, except for the use of lung function for school-aged children and youths. Impairment includes an assessment of the patient’s recent symptom frequency and intensity and functional limitations (ie, daytime symptoms, nighttime awakenings, need for short-acting β2-agonists for quick relief, work or school days missed, ability to engage in normal or desired activities, and quality-of-life assessments) and airflow compromise preferably using spirometry. On the other hand, risk refers to an evaluation of the patient’s likelihood of developing asthma exacerbations, reduced lung growth in children (or progressive decline in lung function in adults), or risk of untoward effects from medications.
Education is important and partnership with the child’s family is a key component in the management to improve adherence and outcomes. The patient and family must understand the role of asthma triggers, the importance of disease activity even without obvious symptoms, how to use objective measures to gauge disease activity, and the importance of airway inflammation—and they must learn to recognize the warning signs of worsening asthma, allowing for early intervention. A stepwise care plan should be developed for all patients with asthma. Providing asthma action plans is currently a requirement that is tracked by many hospitals and others to document that educational instruction for chronic disease management has been given. Asthma action plans should be provided to school personnel and all those who care for children with asthma.
Because the degree of airflow limitation is poorly perceived by many patients, peak flow meters can aid in the assessment of airflow obstruction and day-to-day disease activity. Peak flow rates may provide early warning of worsening asthma. They are also helpful in monitoring the effects of medication changes. Spacer devices optimize delivery of medication from metered-dose inhalers (MDIs) to the lungs and, with inhaled steroids, minimize side effects. Large-volume spacers are preferred. Poor understanding by patients and families of proper device use can lead to inadequate delivery and treatment with inhaled medications, especially inhaled controllers. Short instructive videos for device use can be provided to educate families and other caregivers (URL: http://www.thechildrenshospital.org/conditions/lung/asthmavideos.aspx).
Patients should avoid exposure to tobacco smoke and allergens to which they are sensitized, exertion outdoors when levels of air pollution are high, β-blockers, and sulfite-containing foods. Patients with persistent asthma should be given the inactivated influenza vaccine yearly unless they have a contraindication.
For patients with persistent asthma, the clinician should use the patient’s history to assess sensitivity to seasonal allergens and Alternaria mold; use in vitro testing (either by skin or blood test) to assess sensitivity to perennial indoor allergens; assess the significance of positive tests in the context of the patient’s history; and identify relevant allergen exposures. For dust mite–allergic children, important environmental control measures include encasing the pillow and mattress in an allergen-impermeable cover and washing the sheets and blankets on the patient’s bed weekly in hot water. Other measures include keeping indoor humidity below 50%, minimizing the number of stuffed toys, and washing such toys weekly in hot water. Children allergic to furred animals or feathers should avoid indoor exposure to pets, especially for prolonged periods of time. If removal of the pet is not possible, the animal should be kept out of the bedroom with the door closed. Carpeting and upholstered furniture should be removed. While a high-efficiency particle-arresting filter unit in the bedroom may reduce allergen levels, symptoms may persist if the pet remains indoors. For cockroach-allergic children, control measures need to be instituted when infestation is present in the home. Poison baits, boric acid, and traps are preferred to chemical agents, which can be irritating if inhaled by asthmatic individuals. Indoor molds are especially prominent in humid or damp environments. Measures to control dampness or fungal growth in the home may be of benefit. Patients can reduce exposure to outdoor allergens by staying in an air-conditioned environment. Allergen immunotherapy may be useful for implicated aeroallergens that cannot be avoided. However, it should be administered only in facilities staffed and equipped to treat life-threatening reactions.
Patients should be treated for rhinitis, sinusitis, or gastroesophageal reflux, if present. Treatment of upper respiratory tract symptoms is an integral part of asthma management. Intranasal corticosteroids are recommended to treat chronic rhinosinusitis in patients with persistent asthma because they reduce lower airway hyperresponsiveness and asthma symptoms. Intranasal cromolyn reduces asthma symptoms during the ragweed season but less so than intranasal corticosteroids. Treatment of rhinosinusitis includes medical measures to promote drainage and the use of antibiotics for acute bacterial infections (see Chapter 18). Medical management of gastroesophageal reflux includes avoiding eating or drinking 2 hours before bedtime, elevating the head of the bed with 6- to 8-in blocks, and using appropriate pharmacologic therapy.
2. Pharmacologic therapy—A revised stepwise approach to pharmacologic therapy, broken down by age categories, is recommended in the NAEPP EPR3 (http://www.nhlbi.nih.gov) (Table 38–4). This approach is based on the concepts of asthma severity and asthma control. A separate set of recommendations for younger children is provided given the lack of tools which can be used to assess lung function and quality of life otherwise available for older children. Treatment recommendations for older children and adults are better supported by stronger evidence from available clinical trials, whereas those for younger children have been extrapolated from studies in older children and adults.
Table 38–4. Stepwise approach for managing asthma in children.
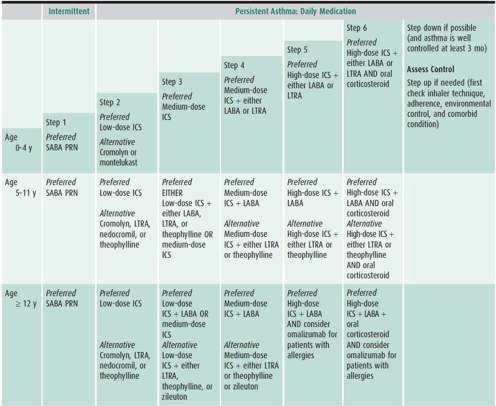

The choice of initial therapy is based on assessment of asthma severity. For patients who are already on controller therapy, treatment can be adjusted based on assessment of asthma control and responsiveness to therapy. The goals of therapy are to reduce the components of both impairment (eg, preventing chronic and troublesome symptoms, allowing infrequent need of quick-relief medications, maintaining “normal” lung function, maintaining normal activity levels including physical activity and school attendance, meeting families’ expectations and satisfaction with asthma care) and risk (eg, preventing recurrent exacerbations, reduced lung growth, and medication adverse effects).
1. The stepwise approach is meant to assist, not replace, the clinical decision making required to meet individual patient needs.
2. In the absence of persistent symptoms, the new clinical guidelines recommend considering initiation of long-term controller therapy for infants and younger children who have risk factors for asthma (ie, modified asthma predictive index: parental history of asthma, physician-diagnosed atopic dermatitis, or sensitization to aeroallergens or two of the following: wheezing apart from colds, sensitization to foods, or peripheral eosinophilia) and four or more episodes of wheezing over the past year that lasted longer than 1 day and affected sleep or two or more exacerbations in 6 months requiring systemic corticosteroids.
3. Inhaled corticosteroids, either as monotherapy or in combination with adjunctive therapy, are preferred treatment for all levels of persistent asthma.
4. Along with medium-dose inhaled corticosteroids, combination therapy with inhaled corticosteroids plus any of the following adjunctive therapies—long-acting inhaled β2-agonists (LABAs), leukotriene modifying agents, cromones, and theophylline—is recommended as step 3 treatment for moderate persistent asthma, or as step-up therapy for uncontrolled persistent asthma for school-aged children and youths. In children aged 0–4 years, medium-dose inhaled corticosteroids as monotherapy remain the step 3 therapy, and combination therapy to be initiated only as a step 4 treatment. A rescue course of systemic corticosteroids may be necessary at any step.
Asthma medications are classified as long-term controller medications and quick-relief medications. The former includes anti-inflammatory agents, leukotriene modifiers, and long-acting bronchodilators. Although LABAs (salmeterol, formoterol) are β-agonists, they are considered to be daily controller medications, but unlike the other asthma controller medications with primarily anti-inflammatory properties, LABAs cannot be administered as monotherapy.
Inhaled corticosteroids are the most potent inhaled anti-inflammatory agents currently available. Different inhaled corticosteroids are not equivalent on a per puff or microgram basis (Table 38–5). Early intervention with inhaled corticosteroids can improve asthma control and prevent exacerbations during treatment, but they do not prevent the development of persistent asthma nor do they alter its natural history. Long-term inhaled corticosteroids may be associated with early slowing of growth velocity in children, and although this can impact the final adult height by a minimum degree, it is not a cumulative effect. Possible risks from inhaled corticosteroids need to be weighed against the risks from undertreated asthma. The adverse effects from inhaled corticosteroids are generally dose and duration dependent, so that greater risks for systemic adverse effects are expected with high doses. The various inhaled corticosteroids are delivered in different devices such as MDI (beclomethasone, ciclesonide, fluticasone, flunisolide, and triamcinolone), dry powder inhaler (DPI) (fluticasone [Diskus], budesonide [Flexhaler], and mometasone [Twisthaler]), and nebulized aerosol suspensions (budesonide respules). Inhaled medications delivered in MDI now use the more ozone friendly hydrofluoroalkane (HFA) propellant, which has replaced chlorofluorocarbons (CFC). See instructions for different device use at the following URL: http://www.thechildrenshospital.org/conditions/lung/asthmavideos.aspx.
Table 38–5. Estimated comparative inhaled corticosteroid doses.
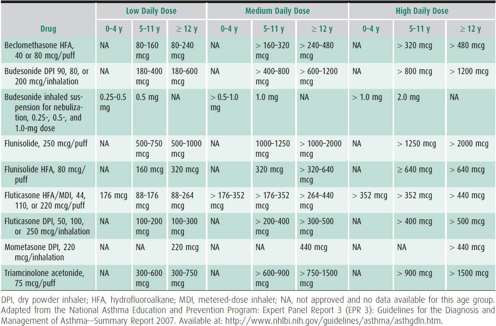
Only inhaled corticosteroids have been shown to be effective in long-term clinical studies with infants. Nebulized budesonide is approved for children as young as 12 months. The suspension (available in quantities of 0.25 mg/2 mL, 0.5 mg/2 mL, and 1.0 mg/2 mL) is usually administered either once or twice daily in divided doses. For effective drug delivery, it is critical that the child has a mask secured on the face for the entire treatment, as blowing it in the face is not effective and yet a common practice by parents. Notably, this drug should not be given by ultrasonic nebulizer. Limited data suggest that inhaled corticosteroids may be effective even in very young children when delivered by MDI with a spacer and mask.
Fewer data are available with nedocromil, although data from the Childhood Asthma Management Program study showed that an inhaled corticosteroid was superior to nedocromil with respect to several efficacy parameters, including rate of hospitalization, symptom-free days, need for albuterol rescue, and longer time to treatment with prednisone, when each was compared to a placebo.
Theophylline is rarely used. Sustained-release theophylline, an alternative long-term control medication for older children, may have particular risks of adverse effects in infants, who frequently have febrile illnesses that increase theophylline concentrations. Hence, if theophylline is used, it requires monitoring of serum concentration to prevent numerous dose-related acute toxicities.
For school aged children whose asthma is uncontrolled on low dose inhaled corticosteroid (ie, requiring step 3 guidelines therapy), majority are likely to respond to a step up combination therapy with a long-acting β2-agonist bronchodilator (eg, salmeterol and formoterol), although some respond best either to an increased dose of inhaled corticosteroid or to an addition of a leukotriene receptor antagonist. LABAs should not be used for treatment of acute symptoms, nor should they be used without any inhaled corticosteroid therapy, even if the patient feels better. Salmeterol is available as an inhalation powder (one inhalation twice daily for patients aged 4 years and older). It is also available combined with fluticasone (50 mcg salmeterol with 100, 250, or 500 mcg fluticasone in a DPI or 21 mcg salmeterol with 45, 115, or 230 mcg fluticasone in an MDI). For children 12 years and older, one inhalation DPI or two inhalations MDI can be taken twice daily. (Note: The 100/50 fluticasone/salmeterol combination is approved in children aged 4 and older.) Salmeterol can also be used 30 minutes before exercise (but not in addition to regularly used LABAs). Formoterol has a more rapid onset of action and is available singly as a DPI (Aerolizer, 12 mcg) or combined with an inhaled corticosteroid (formoterol fumarate, either 4.5 mcg with budesonide [80 or 160 mcg] or 5 mcg with mometasone [100 or 200 mcg], in an MDI). Formoterol DPI is approved for use in children 5 years and older, one inhalation (12 mcg) twice daily, while the combination product is approved for children 12 years and older, two inhalations twice daily. For long-term control, formoterol should be used in combination with an anti-inflammatory agent. It can be used for exercise-induced bronchospasm in patients 5 years and older, one inhalation at least 15 minutes before exercise (but not in addition to regularly used LABAs). Of note, the U.S. Food and Drug Administration (FDA) has requested the manufacturers of Advair Diskus and HFA (salmeterol and fluticasone), Serevent Diskus (salmeterol xinafoate), Foradil Aerolizer (formoterol fumarate), Symbicort HFA, and Brovana (arformoterol tartrate inhalation solution, a LABA approved for chronic obstructive pulmonary disease) to update their product information warning sections regarding an increase in severe asthma episodes associated with these agents. This action is in response to data showing an increased number of asthma-related deaths in patients receiving LABA therapy in addition to their usual asthma care as compared with patients not receiving LABAs. This notice is also intended to reinforce the appropriate use of LABAs in the management of asthma. Specifically, LABA products should not be initiated as first-line asthma therapy, used with worsening wheezing, or used for acute control of bronchospasm. No data are available regarding safety concerns in patients using these products for exercise-induced bronchoconstriction. Additional information, including copies of the Patient and Healthcare Professional information sheets, can be found at: http://www.fda.gov/cder/drug/infopage/LABA/default.htm.
Montelukast and zafirlukast are leukotriene-receptor antagonists available in oral formulations. Montelukast is given once daily and has been approved for treatment of chronic asthma in children aged 1 year and older, as an alternative step 2 monotherapy and add-on therapy for steps 3–6. It is also indicated for seasonal allergic rhinitis in patients 2 years and older, and for perennial allergic rhinitis in patients 6 months and older. To date, no drug interactions have been noted. The dosage is 4 mg for children 1–5 years (oral granules are available for children aged 12–23 months), 5 mg for children aged 6–14 years, and 10 mg for those aged 15 years and older. The drug is given without regard to mealtimes, preferably in the evening. Zafirlukast is approved for patients aged 5 years and older. The dose is 10 mg twice daily for those 5–11 years and 20 mg twice daily for those 12 years and older. It should be taken 1 hour before or 2 hours after meals. Zileuton is a 5-lipoxygenase inhibitor indicated for chronic treatment in children 12 years of age and older, available in regular 600 mg dose tablet four times a day or extended release 600 mg dose tablet, 2 tablets twice a day. Patients need to have hepatic transaminase levels evaluated at initiation of therapy, then once a month for the first 3 months, every 2–3 months for the remainder of the first year, and periodically thereafter if receiving long-term zileuton therapy. Rare cases of Churg-Strauss syndrome have been reported in adult patients with severe asthma whose steroid dosage was being tapered during concomitant treatment with leukotriene-receptor antagonists (as well as inhaled corticosteroids), but no causal link has been established. Both zafirlukast and zileuton are microsomal P-450 enzyme inhibitors that can inhibit the metabolism of drugs such as warfarin and theophylline. The FDA has requested that manufacturers include a precaution in the drug prescribing information (drug labeling) regarding neuropsychiatric events (agitation, aggression, anxiousness, dream abnormalities and hallucinations, depression, insomnia, irritability, restlessness, suicidal thinking and behavior, and tremor) based on postmarket reports of patients taking leukotriene modifying agents. Of note, in a study of children with mild to moderate persistent asthma that looked at whether responses to an inhaled corticosteroid and a leukotriene-receptor antagonist are concordant for individuals or whether asthmatic patients who do not respond to one medication respond to the other, response to fluticasone and montelukast were found to vary considerably. Children with low pulmonary function or high levels of markers associated with allergic inflammation responded better to the inhaled corticosteroid.
Children with persistent asthma who remain uncontrolled on inhaled corticosteroid monotherapy are more likely to respond to a combination treatment of an inhaled corticosteroid and a LABA; however, there are children who can respond best to a higher dose of inhaled corticosteroid, or even a low dose inhaled corticosteroid plus montelukast. It has not been definitely determined what clinical features would be helpful in selecting the most appropriate medication for any one patient. Recent studies in adults have also shown the efficacy of a long-acting antimuscarinic agent, tiotropium (Spiriva), as an add-on therapy to inhaled corticosteroids.
Quick-relief medications include short-acting inhaled β2-agonists (SABAs) such as albuterol, levalbuterol, pirbuterol, or terbutaline. Albuterol can be given by nebulizer, 0.05 mg/kg (with a minimal dose of 0.63 mg and a maximum of 5 mg) in 2–3 mL saline (although it is also available in a 2.5 mg/3 mL single vial or 5 mg/mL concentrated solution) or by MDI (90 mcg/actuation). It is better to use SABAs as needed rather than on a regular basis. Increasing use, including more than one canister per month, may signify inadequate asthma control and the need to step up or revise controller therapy. Levalbuterol, the (R)-enantiomer of racemic albuterol, is available in solution for nebulization in patients aged 6–11 years, 0.31 mg every 8 hours, and in patients 12 years and older, 0.63–1.25 mg every 8 hours. It has recently become available in an HFA formulation for children 4 years and older, two inhalations (90 mcg) every 4–6 hours as needed. Anticholinergic agents such as ipratropium, 1–3 puffs or 0.25–0.5 mg by nebulizer every 6 hours, may provide additive benefit when used together with an inhaled SABA. Systemic corticosteroids such as prednisone, prednisolone, and methylprednisolone can be given in a dosage of 1–2 mg/kg, usually up to 60 mg/d in single or divided doses for 3–10 days. There is no evidence that tapering the dose following a “burst” prevents relapse.
Anti-IgE (omalizumab) is a recombinant DNA-derived humanized IgG1 monoclonal antibody that selectively binds to human IgE. It inhibits the binding of IgE to the high-affinity IgE receptor (FcεRI) on the surface of mast cells and basophils. Reduction in surface-bound IgE on FcεRI-bearing cells limits the degree of release of mediators of the allergic response. Treatment with omalizumab also reduces the number of FcεRI receptors on basophils in atopic patients. Omalizumab is indicated for patients 12 years of age and older with moderate to severe persistent asthma who have a positive skin test or in vitro reactivity to a perennial aeroallergen with total serum IgE of 30–700 IU/mL, and whose symptoms are inadequately controlled with medium to high dose inhaled corticosteroids. Omalizumab has been shown to decrease the incidence of asthma exacerbations and improve asthma control in these patients. Dosing is based on the patient’s weight and serum IgE level and is given subcutaneously every 2–4 weeks. The FDA has ordered a black box warning to the label because of new reports of serious and life-threatening anaphylactic reactions (bronchospasm, hypotension, syncope, urticaria, and angioedema of the throat or tongue) in patients after treatment with omalizumab (Xolair). Based on reports from approximately 39,500 patients, anaphylaxis following omalizumab treatment occurred in at least 0.1% of treated people. Although these reactions occurred within 2 hours of receiving a omalizumab subcutaneous injection, they also included reports of serious delayed reactions 2–24 hours or even longer after receiving the injections. Anaphylaxis occurred after any dose of omalizumab (including the first dose), even in patients with no allergic reaction to previous doses. Omalizumab-treated patients should be observed in the facility for an extended period after the drug is given, and medical providers who administer the injection should be prepared to manage life-threatening anaphylactic reactions. Patients who receive omalizumab should be fully informed about the signs and symptoms of anaphylaxis, their chance of developing delayed anaphylaxis following each injection, and how to treat it, including the use of autoinjectable epinephrine. The small risks of malignant neoplasms (a variety of types, e.g., breast, nonmelanoma skin, prostate, melanoma, and parotid) in clinical studies of adults and adolescents (≥ 12 years of age) with asthma and other allergic disorders were reported in 20 of 4127 (0.5%) omalizumab-treated patients compared to 5 of 2236 (0.2%) controls.
Immunotherapy (discussed in more detail in a subsequent section) can be considered for children 5 years and older with allergic asthma who require steps 2, 3, and 4 therapy.
Continual monitoring is necessary to ensure that control of asthma is achieved and sustained. Once control is established, gradual reduction in therapy is appropriate and may help determine the minimum amount of medication necessary to maintain control. Regular follow-up visits with the clinician are important to assess the degree of control and consider appropriate adjustments in therapy. At each step, patients should be instructed to avoid or control exposure to allergens, irritants, or other factors that contribute to asthma severity.
Referral to an asthma specialist for consultation or co-management is recommended if there are difficulties in achieving or maintaining control. For children younger than age 5 years, referral is recommended for moderate persistent asthma or if the patient requires step 3 or 4 care and should be considered if the patient requires step 2 care. For children 5 years and older, consultation with a specialist is recommended if the patient requires step 4 care or higher and should be considered at step 3. Referral is also recommended if allergen immunotherapy or anti-IgE therapy is being considered.
3. Exercise-induced bronchospasm—Exercise-induced bronchospasm should be anticipated in all asthma patients. It typically occurs during or minutes after vigorous activity, reaches its peak 5–10 minutes after stopping the activity, and usually resolves over the next 20–30 minutes. Participation in physical activity should be encouraged in children with asthma, although the choice of activity may need to be modified based on the severity of illness, presence of other triggers such as cold air, and, rarely, confounding factors such as osteoporosis. Poor endurance or exercise-induced bronchospasm can be an indication of poorly controlled persistent asthma. If symptoms occur during usual play activities, either initiation of or a step-up in long-term therapy is warranted. However, for those with exercise-induced bronchospasm as the only manifestation of asthma despite otherwise being “well-controlled,” treatment immediately prior to vigorous activity or exercise is usually effective. SABAs, leukotriene receptor antagonists, cromolyn, or nedocromil can be used before exercise. The combination of a SABA with either cromolyn or nedocromil is more effective than either drug alone. Salmeterol and formoterol may block exercise-induced bronchospasm for up to 12 hours (as discussed earlier). However, decreased duration of protection against exercise-induced bronchospasm can be expected with regular use. Montelukast may be effective up to 24 hours. An extended warm-up period may induce a refractory state, allowing patients to exercise without a need for repeat medications.
B. Acute Asthma
1. General measures—The most effective strategy in managing asthma exacerbations involves early recognition of warning signs and early treatment. For patients with moderate or severe persistent asthma or a history of severe exacerbations, this should include a written action plan. The latter usually defines the patient’s green, yellow, and red zones based on symptoms (and PEFR for patients with poor symptom perception) with corresponding measures to take according to the state the patient is in. PEFR cut-off values are conventionally set as > 80% (green), 50%–80% (yellow), and < 50% (red) of the child’s personal best. Prompt communication with the clinician is indicated with severe symptoms or a drop in peak flow or with decreased response to SABAs. At such times, intensification of therapy may include a short course of oral corticosteroids. The child should be removed from exposure to any irritants or allergens that could be contributing to the exacerbation.
2. Management at home—Early treatment of asthma exacerbations may prevent hospitalization and a life-threatening event. Initial treatment should be with a SABA such as albuterol or levalbuterol; 2–6 puffs from an MDI can be given every 20 minutes up to three times, or a single treatment can be given by nebulizer (0.05 mg/kg [minimum dose, 1.25 mg; maximum, 2.5 mg] of 0.5% solution of albuterol in 2–3 mL saline; or 0.075 mg/kg [minimum dose, 1.25 mg; maximum, 5 mg] of levalbuterol). If the response is good as assessed by sustained symptom relief or improvement in PEFR to over 80% of the patient’s best, the SABA can be continued every 3–4 hours for 24–48 hours. Patients should be advised to seek medical care once excessive doses of bronchodilator therapy are used or for prolonged periods (eg, > 12 puffs/d for > 24 hours). Doubling the dose of inhaled corticosteroids is not proven sufficient to prevent worsening of exacerbations; however, recent evidence indicates that quadrupling the inhaled corticosteroid dose at the early sign of deterioration might be effective. If the patient does not completely improve from the initial therapy or PEFR falls between 50% and 80% predicted or personal best, the SABA should be continued, an oral corticosteroid should be added, and the patient should contact the physician urgently. If the child experiences marked distress or if PEFR persists at 50% or less, the patient should repeat the SABA immediately and go to the emergency department or call 911 or another emergency number for assistance.
3. Management in the office or emergency department—Functional assessment of the patient includes obtaining objective measures of airflow limitation with PEFR or FEV1 and monitoring the patient’s response to treatment; however, very severe exacerbations and respiratory distress may prevent the execution of lung function measurements using maximal expiratory maneuver. Flow-volume loops should be obtained to differentiate upper and lower airway obstruction, especially in patients with atypical presentation. Other tests may include oxygen saturation and blood gases. Chest radiographs are not recommended routinely but should be considered to rule out pneumothorax, pneumomediastinum, pneumonia, or lobar atelectasis. If the initial FEV1 or PEFR is over 40%, initial treatment can be with a SABA by inhaler (albuterol, 4–8 puffs) or nebulizer (0.15 mg/kg of albuterol 0.5% solution; minimum dose, 2.5 mg), up to three doses in the first hour. Oxygen should be given to maintain oxygen saturation at greater than 90%. Oral corticosteroids (1–2 mg/kg/d in divided doses; maximum of 60 mg/d for children aged ≤ 12 years and 80 mg/d for those > 12 years) should be instituted if the patient responds poorly to therapy or if the patient has recently been on oral corticosteroids. Sensitivity to adrenergic drugs may improve after initiation of corticosteroids. For severe exacerbations or if the initial FEV1 or PEFR is under 40%, initial treatment should be with a high-dose SABA plus ipratropium bromide, 1.5–3 mL every 20 minutes for 3 doses (each 3 mL vial contains 0.5 mg ipratropium bromide and 2.5 mg albuterol), then as needed by nebulizer. Continuous albuterol nebulized treatments (0.5 mg/kg/hour for small and 10–15 mg/hour for older children) can be administered for evidence of persistent obstruction. Oxygen should be given to maintain oxygen saturation at greater than 90%, and systemic corticosteroids should be administered. For patients with severe exacerbation having no response to initial aerosolized therapy, or for those who cannot cooperate with or who resist inhalation therapy, adjunctive therapies such as intravenous magnesium sulfate (25–75 mg/kg up to 2 g in children) and heliox-driven albuterol nebulization should be considered. Epinephrine 1:1000 or terbutaline 1 mg/mL (both 0.01 mg/kg up to 0.3–0.5 mg) may be administered subcutaneously every 20 minutes for three doses; although the use of intravenous β2-agonists is still unproven. For impending or ongoing respiratory arrest, patients should be intubated and ventilated with 100% oxygen, given intravenous corticosteroids, and admitted to an intensive care unit (ICU). Potential indications for ICU admission also include any FEV1 or PEFR less than 25% of predicted that improves less than 10% after treatment or values that fluctuate widely. (See Asthma [life-threatening] in Chapter 14.) Further treatment is based on clinical response and objective laboratory findings. Hospitalization should be considered strongly for any patient with a history of respiratory failure.
4. Hospital management—For patients who do not respond to outpatient and emergency department treatment, admission to the hospital becomes necessary for more aggressive care and support. The decision to hospitalize should also be based on presence of risk factors for mortality from asthma, duration and severity of symptoms, severity of airflow limitation, course and severity of previous exacerbations, medication use at the time of the exacerbation, access to medical care, and home and psychosocial conditions. Fluids should be given at maintenance requirements unless the patient has poor oral intake secondary to respiratory distress or vomiting, because overhydration may contribute to pulmonary edema associated with high intrapleural pressures generated in severe asthma. Potassium requirements should be kept in mind because both corticosteroids and β2-agonists can cause potassium loss. Moisturized oxygen should be titrated by oximetry to maintain oxygen saturation above 90%. Inhaled β2-agonist should be continued by nebulization in single doses as needed or by continuous therapy, along with systemic corticosteroids (as discussed earlier). Ipratropium is no longer recommended during hospitalization. In addition, the role of methylxanthines in hospitalized children remains controversial. Antibiotics may be necessary to treat coexisting bacterial infection. Sedatives and anxiolytic agents are contraindicated in severely ill patients owing to their depressant effects on respiration. Chest physiotherapy is usually not recommended for acute exacerbations.
5. Patient discharge—Criteria for discharging patients home from the office or emergency department should include a sustained response of at least 1 hour to bronchodilator therapy with FEV1 or PEFR greater than 70% of predicted or personal best and oxygen saturation greater than 90% in room air. Prior to discharge, the patient’s or caregiver’s ability to continue therapy and assess symptoms appropriately needs to be considered. Patients should be given an action plan for management of recurrent symptoms or exacerbations, and instructions about medications should be reviewed. The inhaled SABA and oral corticosteroids should be continued, the latter for 3–10 days. Finally, the patient or caregiver should be instructed about the follow-up visit, typically within 1 week. Hospitalized patients should receive more intensive education prior to discharge. Referral to an asthma specialist should be considered for all children with severe exacerbations or multiple emergency department visits or hospitalizations.
 Prognosis
Prognosis
Since the 1970s, morbidity rates for asthma have increased, but mortality rates may have stabilized. Mortality statistics indicate that a high percentage of deaths have resulted from underrecognition of asthma severity and undertreatment, particularly in labile asthmatic patients and in asthmatic patients whose perception of pulmonary obstruction is poor. Long-term outcome studies suggest that children with mild symptoms generally outgrow their asthma, while patients with more severe symptoms, marked airway hyperresponsiveness, and a greater degree of atopy tend to have persistent disease. Data from an unselected birth cohort from New Zealand showed more than one in four children had wheezing that persisted from childhood to adulthood or that relapsed after remission. Recent evidence suggests that early intervention with anti-inflammatory therapy does not alter the development of persistent asthma, and it is also unclear if such intervention or environmental control measures influence the natural history of childhood asthma. Nonetheless, the pediatrician or primary care provider together with the asthma specialist has the responsibility to optimize control and, it is hoped, reduce the severity of asthma in children. Interventions that can have long-term effects such as halting progression or inducing remission are necessary to decrease the public health burden of this common condition.
Resources for healthcare providers, patients, and families include:
Asthma and Allergy Foundation of America
1233 20th St NW, Suite 402
Washington, DC 20036; (800) 7-ASTHMA
Asthma and Allergy Network/Mothers of Asthmatics
2751 Prosperity Avenue, Suite 150
Fairfax, VA 22031; (800) 878-4403
Asthma Device Training: http://www.thechildrenshospital.org/conditions/lung/asthmavideos.aspx
Agency for Healthcare Research and Quality. 2006 Hospital
Discharges by age groups.
http://hcupnet.ahrq.gov/. Accessed May 15, 2009.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


