Risk stratification
Factors
Treatment
Low risk
Stage IA, grade I–II
Observation
Intermediate risk
Stage IA, grade III; stage IB, grade I–II
Vaginal brachytherapy (VBT)
High risk
Stage IB, grade III; stage II
EBRT + VBT
Low Risk
These patients have excellent prognosis. The Cochrane review in 2007 concluded that pelvic radiation has deleterious effects on survival in low-risk patients [23]. VBT has not affected survival or recurrence rates compared to observation alone. Hence, observation after surgery is recommended for this group of patients.
Intermediate Risk
Based on the results of the three major randomized trials [8, 11, 15], adjuvant brachytherapy alone should be offered to all intermediate-risk patients. Brachytherapy showed equivalent results in terms of local, locoregional survival to pelvic radiation and significant reduction in radiation-related toxicities.
PORTEC 1 study with 15-year follow-up and GOG 99 study suggested a significant improvement in locoregional control rates of approximately 10 % with the use of postoperative adjuvant radiation (EBRT) without any overall survival benefit. Also, the morbidity associated with adjuvant EBRT was significantly higher especially GI complications. The GI complications were 20 %, mainly diarrhea, abdominal cramps, and frequency of bowel movements. GU symptoms were urgency, frequency, and mild incontinence [24]. PORTEC 2 study suggested that adjuvant EBRT can be replaced by VBT without compromising the outcomes and significant reduction in toxicities and better QOL as reported by patients [25].
High Risk
Pelvic radiation with or without brachytherapy is the recommended treatment.
In a subgroup analysis by Aalders et al. in high-risk group patients, pelvic radiation with brachytherapy had lower rates of cancer-specific deaths (18 % vs. 31.4 %) and pelvic and vaginal recurrences (4.8 % vs. 19.6 %) as compared to brachytherapy alone [12].
Prophylactic vaginal brachytherapy is considered after EBRT in the case of cervical involvement (stage II).
Role of Adjuvant Chemotherapy/Chemoradiotherapy in High-Risk Patients
In high-risk patients even after adequate therapy, 88 % of all recurrences are distant failures, and less than 30 % develop local recurrences. Hence, adjuvant chemotherapy, radio-chemotherapy, etc., have been attempted. The NSGO EC 9501 and MaNGO ILIADE studies evaluated adjuvant radiotherapy versus adjuvant chemoradiotherapy in high-risk women. Their pooled data showed an improvement in 5-year failure-free survival of 13 % from 71 % to 84 % [26].
Currently, there are ongoing prospective phase III randomized trials to evaluate the role of adjuvant chemotherapy and radiotherapy like PORTEC 3, GOG 249, and GOG 248. Completion of these studies and mature data is essential to establish further the role of adjuvant chemo-/radiotherapy in high-risk endometrial cancer patients.
Patients Undergoing Inadvertent Surgery or Incomplete Surgical Staging
In patients who undergo incomplete surgical staging/incidental finding of endometrial cancer, an attempt should be made to stratify them into various risk stratification categories. All patients are usually subjected to imaging at least CT of the abdomen and pelvis to rule out residual disease in the abdomen, peritoneal/omental surfaces, pelvic/para-aortic lymph nodal regions, etc. In general, if imaging is negative, then either VBT (in low and intermediate risk) or EBRT ± VBT (in high risk) is offered. If there are positive findings on imaging, surgical re-exploration for appropriate staging and further adjuvant therapy is offered.
Low-Risk Patients
Observation or VBT alone
Intermediate-Risk Patients
EBRT ± VBT (imaging negative) or surgical re-exploration (imaging positive)
High-Risk Patients
EBRT ± VBT (imaging negative) or surgical re-exploration (imaging positive) and adjuvant therapy according to final staging
Other Histologies: Clear Cell, Papillary Serous, and Carcinosarcomas
Stage IA: Observation or chemotherapy or pelvic RT
Stage IB–II: Chemotherapy with or without pelvic RT ± VBT
Radiation Therapy for Endometrial Cancer
External Radiation Planning
In post-operative settings, aim is to treat upper 3 cm of vagina including vault, para-vaginal soft tissues and draining lymph node regions including common, external, and internal iliac lymph node regions [Clinical Target Volume (CTV)] and achieve optimal sparing of various neighboring structures like bladder, small bowel, rectum, bone marrow, and the femoral heads.
Conventional
The radiation portal is usually two-field (AP/PA) or four-field (box) arrangement. Shielding to minimize dose to neighboring structures (bowel, bladder, and rectum) can be used. The radiation portals are usually marked using fluoroscopy/CT guidance. The radiation field is fixed based on bony landmarks with superior border at L5-S1 and inferior border below the obturator canal to include upper 1/2–1/3 of the vagina, and the lateral borders are placed at 1.5–2 cm lateral to the pelvic brim for two-field AP/PA technique. Dose is prescribed in the midplane (Fig. 22.1).
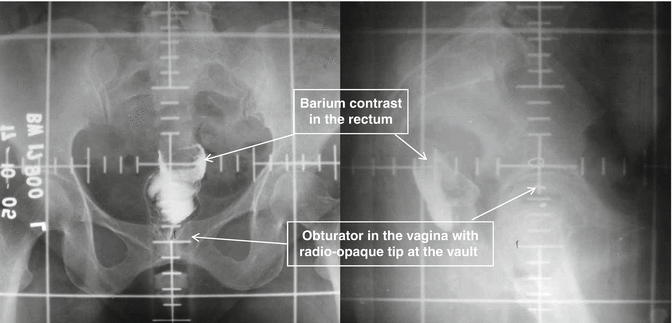

Fig. 22.1
Anteroposterior and lateral EBRT planning X-ray illustrating standard pelvic portals marked by lead wires and scale
In the four-field technique (AP/PA and bilateral), apart from AP/PA portals as described above, two additional lateral (right and left) fields with anterior border anterior to pubic symphysis and posterior border at S3 are placed. Customized blocks to shield the small bowel anterosuperiorly and the low ano-rectum inferiorly to reduce radiation toxicities are attempted (Fig. 22.1).
Conformal and Newer Techniques
More conformal techniques like three-dimensional conformal radiation techniques (3DCRT) and intensity-modulated radiation therapy (IMRT) are used to reduce normal tissue toxicities. These techniques involve the use of CT imaging, delineation of various targets and organs, planning, and dosimetric evaluation. 3DCRT uses fixed beams shaped to the target volume with uniform dose intensity, while IMRT uses optimized nonuniform dose intensities to deliver conformal radiation to the target to minimize doses to surrounding normal tissues. IMRT studies have shown a significant reduction in doses to the small bowel, bladder, and rectum which translated into reduction in radiation-related acute and chronic toxicities as compared to conventional/3DCRT techniques [27, 28, 29].
Various guidelines are available which define in detail concepts and contouring of the target and OARs [30] (Fig. 22.2).
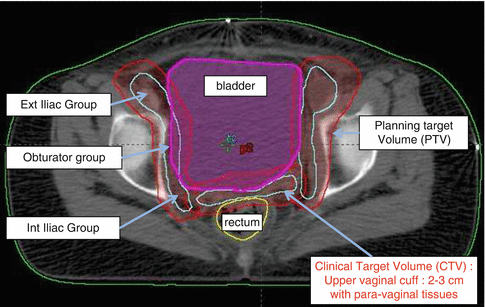

Fig. 22.2
Representative axial CT slice showing various target volumes (CTV and PTV) and organ definitions for conformal/IMRT planning
In a retrospective review by Shih et al., the outcomes with postoperative pelvic IMRT in high-risk endometrial cancer were excellent with 5-year DFS and OS >88 % with a favorable toxicity profile [31]. The preliminary data from phase II RTOG 0418 trial highlights the advantages of IMRT. Forty-three patients of endometrial cancer (93 % stage I–II) treated with adjuvant IMRT showed a 3-year DFS and OS rates of 91 % and 92 %, respectively [32] (Fig. 22.3).
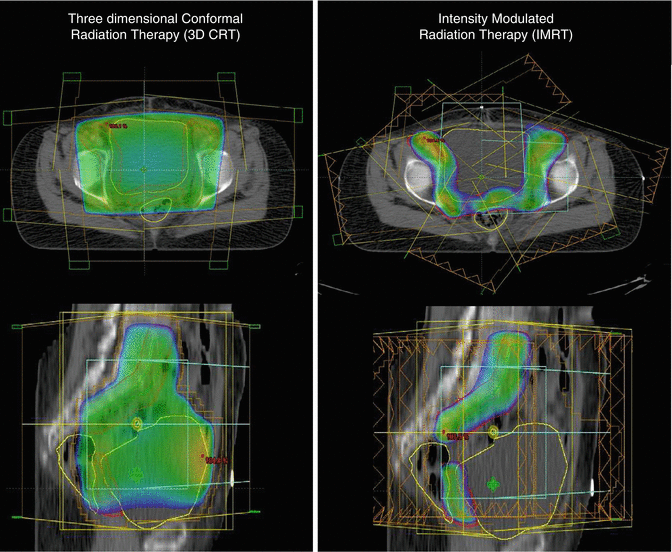

Fig. 22.3
Axial and sagittal CT comparison of dose distribution: 3DCRT (left panel) versus IMRT plan (right panel) illustrating sparing of organs at risk with IMRT
External Radiation Doses
An external radiation dose of 45–50.4 Gy in 25–28 fractions in 5–5.5 weeks is recommended.
Brachytherapy
Vaginal brachytherapy (VBT) is effective with <5 % recurrence rates and low toxicity. Care should be taken to choose the appropriate applicator to adequately treat the vaginal mucosa. Adjuvant brachytherapy is performed at least 4 weeks after surgery. Vaginal cylinders or ovoids are commonly used. The aim is to radiate proximal 3–5 cm of the vagina including the vault.
Vaginal cylinders are the standard applicators used. They are available with a diameter of 20, 25, or 30 mm. According to ABS guidelines for vaginal brachytherapy, the size of the cylinder should be carefully chosen to ensure good contact between the cylinder and the vaginal mucosa [33]. The largest size which fits snugly to the vagina should be chosen. This is to avoid any air gaps and prevent underdosage of the vaginal mucosa. Radiopaque markers or clips may be placed at the vaginal apex to confirm that the applicator is in contact with the mucosa. The length of the vagina should be measured from the vault to the level of the introitus. Plain X-ray film- or CT imaging-based planning is done. The proximal 3–5 cm of the vagina with 5 mm thickness is treated with VBT. The dose should be prescribed to a depth of 5 mm from the vaginal mucosa or at the vaginal surface. More than 95 % of lymphatic channels are located in the first 3 mm of the mucosa. Brachytherapy can be delivered with high dose rate (HDR) or low dose rate (LDR) and rarely with pulse dose rate (PDR).
Brachytherapy Doses
LDR prescription is 50–60 Gy to the surface over 60–70 h when used alone. However, HDR brachytherapy is preferable. The common fractionation schedules used are, for brachytherapy alone, 4 Gy × 6, 6 Gy × 5, 7 Gy × 4, and 8 Gy × 3 at the vaginal surface or 7Gy × 3 fractions at 5 mm depth when VBT alone is planned. If VBT boost is planned after EBRT, 4Gy × 4 and 6 Gy × 3 at the vaginal surface or 5 Gy × 3 fractions or 6 Gy × 3 fractions at 5 mm are delivered (Fig. 22.4).
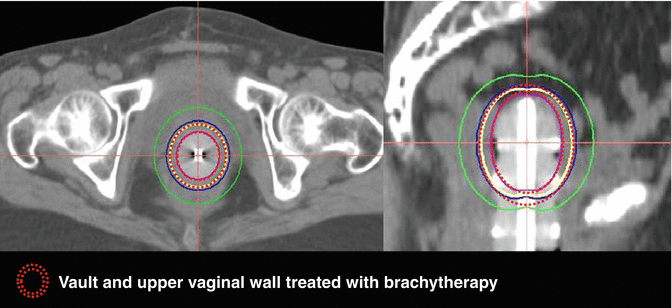

Fig. 22.4
Axial and sagittal CT images showing intravaginal brachytherapy cylinders, brachytherapy doses to vault, and upper vaginal wall
Clinical Outcome and Survival
Patients with early endometrial cancer have excellent outcomes, with 5-year disease-free and overall survivals of 93 % and 98 %, respectively, for stage I patients and 70–80 % for stage II [34]. The survival outcomes according to risk stratification for early endometrial cancers are as follows:
Low Risk
The risk of recurrence is low, with 5-year cancer-specific and overall survival of 98 % and 96 %, respectively, and progression-free survival in the range from 90 % to 99 % [19].
Intermediate Risk
Morbidity of Adjuvant Radiation Therapy
The side effects of pelvic radiation are due to irradiation of the small bowel, bladder, rectosigmoid, femoral heads, and bone marrow which are in close proximity to the target volume. The toxicities seen depend on the tolerance of these organs. Reactions are broadly classified acute and late. The frequency and severity depend on the total dose, dose fractionation, volume of the organ irradiated, concurrent chemotherapy, previous abdominal surgery, and presence of other comorbidities like diabetes, hypertension, or inflammatory bowel disease. The toxicities especially in the small bowel are more pronounced due to small bowel adhesions in the true pelvis and receive relatively high-dose gradients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


