Acute Inflammation
Clifford W. Bogue
Acute inflammation is a coordinated, adaptive response that is triggered by a number of noxious stimuli and conditions, the most prominent being microbial infection and tissue injury.1 The major function of acute inflammation is to provide protection against infection and/or to repair tissue damage. Thus, the process must be carefully regulated in order to be beneficial. A successful acute inflammatory response leads to the elimination of the invading organisms followed by a period of resolution and repair. In certain cases, such as septic shock, acute inflammation becomes dysregulated and can result in extensive tissue damage. Considerable progress has been made in elucidating the molecular mechanisms involved in the acute inflammatory response to microbial pathogens and, to a somewhat lesser extent, to tissue injury. Although there are other triggers to inflammation, much less is known about them and they will not be discussed here. Regardless of the trigger, one key fact is that the hallmarks of inflammation that are commonly associated with microbial infection (ie, redness [rubor], heat [calor], pain [dolor], and swelling [tumor]) are initiated by the innate immune response. Although the cells of the adaptive immune response can contribute to and amplify these effects, the primary signals that initiate and ultimately resolve acute inflammation are linked to innate immune recognition. Of the two branches of the immune system, innate and adaptive, innate immunity is the most ancient and exists, in some form, in all metazoans. Cells of the innate immune system utilize a collection of genomically encoded invariant receptors to recognize the presence of microbial pathogens and signal their presence.2 The role of these mechanisms is becoming increasingly recognized in a large variety of disease conditions and therefore the pediatric clinician benefits from a summary understanding of their biological basis. This chapter is intended to provide this basis through a discussion of the signals that are involved in the initiation of the inflammatory response, the mediators and effectors of the inflammatory response, as well as the factors involved in the transition from inflammation to resolution and tissue remodeling. For a further discussion of the development of the immune system see Chapter 186, and for a discussion of bacterial pathogenesis, bacteremia and septic shock see Chapters 222 and 223.
INDUCTION OF INFLAMMATION VIA PATTERN RECOGNITION
As stated previously, the predominant trigger of acute inflammation is the recognition of microbes by the receptors of the innate immune system. In contrast to the adaptive immune system, which uses the vast repertoire of B- and T-cell antigen receptors to potentially recognize almost any target, the specificities of the innate immune system are “hardwired,” meaning that their microbial targets are selected evolutionarily over time and are, therefore, much more limited in their scope.3 Thus, the innate immune system has evolved a strategy of “pattern recognition” that involves targeting highly conserved features of microbes for detection, enabling receptors of the innate system to detect the wide range of microbial diversity.4,5 The innate receptors that are involved in this process are called pattern recognition receptors (PRRs). PRRs exist as both transmembrane receptors and as cytosolic nucleic acid receptors. The best-characterized PRRs are the Toll-like receptors (TLRs). The founding member of the TLR family is the Drosophila protein Toll, which was originally identified through its ability to control dorsoventral patterning in fruit fly embryos.6 Subsequent analysis demonstrated that flies with Toll mutations have a markedly suppressed ability to produce a key antifungal peptide, as well as reduced survival in the face of fungal infection. To date, a total of 10 human TLRs have been identified, and distinct pathogen-associate molecular patterns (PAMPs) have been identified for several of them.4 Consistent with this role, they are expressed by cells providing the initial line of host defense, including neutrophils, macrophages, dendritic cells, and certain epithelial cells, and they link the recognition of conserved components of bacteria, viruses, fungi, and protozoa to the activation of these cells.5 In addition, with the exception of TLR9, which exists in the cytoplasm, they are all transmembrane molecules (Fig. 115-1). The extracellular amino termini of TLRs have variable leucine-rich repeat (LRR) domains that are involved in the recognition of pathogen-associated molecular patterns and the intracellular domains contain a conserved Toll/interleukin-1 (IL-1) receptor (TIR) domain. The TIR domain, a defining characteristic of the Toll/IL-1 receptor superfamily, is critical in the association of the receptors with downstream signaling molecules that mediate the response to Toll-like receptor stimulation. There is a growing list of ligands for the various TLRs. The list includes lipopolysaccharide (LPS), a component of the cell wall of gram-negative bacteria; peptidoglycan, lipoteichoic acid, and zymosan, components of gram positive bacterial cell walls; double-stranded RNA; flagellin; single-stranded RNA; and nonmethylated CpG motifs in DNA.7
In addition to the large family of transmembrane toll-like receptors, there is a large group of cytosolic pattern recognition receptors that are more varied in their structure and function than the TLRs (Fig. 115-1). One group of these receptors is collectively referred to as nucleotide-binding oligomerization domain-like (Nod-like) receptors (NLRs) based on their structural similarity to the Nod1 and Nod2 proteins.2 The role that this large family plays in inflammation and host defense is an area of significant research with many unanswered questions. The function of Nod1 and Nod2 appears to overlap with the Toll-like receptors. These receptors activate NF-κβ, much like Toll-like receptors, and induce the expression of proinflammatory cytokines.8 Additionally, under certain circumstances, Nod proteins can synergize with Toll-like receptor signaling and result in enhanced cytokine production. One subset of the Nod-like receptors family (Nalp and Naip) controls activation of the inflammasome, a multiprotein complex involved in activating caspase-1, a protease that processes the inflammatory cytokine pro-IL-1 into a mature form that is secreted.9 These Nod-like receptor family members act by recognizing and targeting patterns on bacteria and other microbial pathogens that are similar to those patterns recognized by Toll-like receptors.9 Some of the ligands that have been implicated in the activation of these receptors include bacterial RNA, uric acid crystals, bacterial toxins, and flagellin. Interestingly, these receptors only transduce an inflammatory signal if the ligand is intracellular.
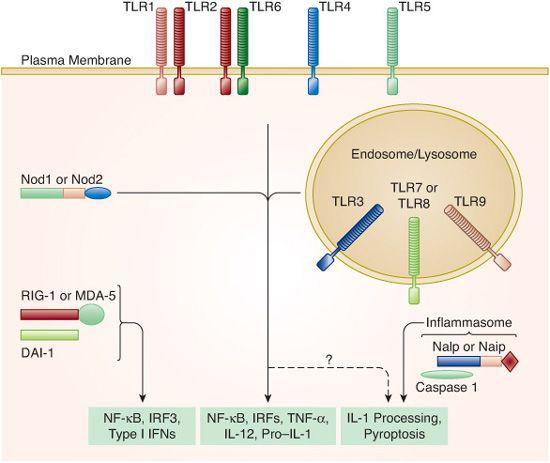
FIGURE 115-1. Pattern recognition by the innate immune system. Shown is a schematic representation of the pattern-recognition receptor (PRR) families within the innate immune system. Toll-like receptors (TLRs) are transmembrane receptors, whereas Nods, Naips, and Nalps, as well as the nucleic acid sensors RIG-1, MDA-5, and DAI-1, are all present in the cytosol. Most of these pattern-recognition receptor families activate common transcription factors and induce the expression of proinflammatory genes. Members of the Nalp and Naip families control activation of the inflammasome, a multiprotein complex responsible for the processing and secretion of IL-1. (Source: Reprinted from Barton GM. J Clin Invest. 2008;118(2):413-420, with permission from the American Society for Clinical Investigation.)
A second group of intracellular pattern recognition receptors is involved in sensing nucleic acids (Fig. 115-1). This group includes retinoic acid–inducible gene I (RIG-I) and the receptors in this group are collectively known as RIG-I-like receptors (RLRs). RIG-I and MDA-5 detect foreign RNA and the receptor DAI detects foreign DNA in the cytosol.10,11 Both the RNA and DNA sensing pathways induce type I interferons (IFNs), thereby activating many of the same signaling pathways as the Toll-like receptors.
THE INNATE IMMUNE SYSTEM IN THE INFLAMMATORY RESPONSE TO INFECTION
The inflammatory response to infection has typically been characterized as having four stages: recognition of infection, recruitment of cells to the site of infection, elimination of the microbe, and resolution of inflammation with return to homeostasis.1
 RECOGNITION OF INFECTION
RECOGNITION OF INFECTION
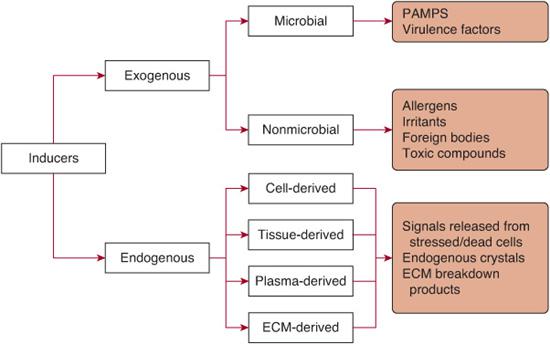
The initial recognition of infection is mediated by multiple cell types resident in the tissues prior to the onset of infection. The two most important cell types playing this role are tissue-resident macrophages and mast cells. As stated previously, these cells (and others) initiate the inflammatory response by recognizing various inducers of inflammation. The inducers of inflammation can be categorized as either exogenous or endogenous (Fig. 115-2). Exogenous inducers may be categorized as either microbial or nonmicrobial. Exogenous microbial inducers include pathogen-associate molecular patterns (PAMPs) and virulence factors. It is important to note that the conserved molecular patterns that make up PAMPs are carried by all microorganisms of a certain class, both pathogenic and commensal.3 Thus, commensal microorganisms that are normally resident in the body, such as those that compose the intestinal microflora, provide an important source of inflammation inducers that are detected by Toll-like receptors.12 The activation of TLRs by commensal bacteria must be actively suppressed. The second type of microbial inducer includes a variety virulence factors and is therefore restricted to pathogens. As outlined above, virulence factors are not sensed directly by TLRs. Rather, they are detected by specialized receptors that include Nod-like receptors and receptors that detect nucleic acids. Exogenous inducers of inflammation that are not microbial in origin include allergens, irritants, foreign bodies, and toxic compounds.
Once the tissue macrophages, dendritic cells, and mast cells have recognized the source of the infection, a variety of inflammatory mediators is produced. The list of mediators includes chemokines, cytokines, vasoactive amines, eicosanoids, and products of proteolytic cascades. Macrophages engulf microbes that they have detected via pattern recognition and the release of proinflammatory cytokines and preformed mediators leads to the second and third steps, which are recruitment of inflammatory cells to the site of infection and elimination of the microbe.
 RECRUITMENT OF INFLAMMATORY CELLS TO THE SITE OF INFECTION AND ELIMINATION OF THE MICROBE
RECRUITMENT OF INFLAMMATORY CELLS TO THE SITE OF INFECTION AND ELIMINATION OF THE MICROBE
The local release of proinflammatory cytokines and other proinflammatory mediators results in changes in the endothelial cells of nearby blood vessels converting infected tissues from a normal to an inflamed state. Cytokines such as TNF-α and IL-1, as well as lipid mediators, activate the nearby endothelium, which results in the selective extravasation of neutrophils and plasma proteins. This selectivity is possible due to the process of inducible ligation of endothelial cell selectins with integrins and chemokine receptors on the surface of white blood cells.13 This process also occurs in the adjacent extravascular space, where newly deposited plasma proteins form a rudimentary matrix for the binding of leukocyte integrins. Usually, neutrophils arrive within a few hours of the beginning of the inflammatory process and monocytes follow somewhat later. Neutrophils contain a battery of mechanisms designed to kill invading organisms via the release of toxic granules, which contain proteases that can produce reactive oxygen and reactive nitrogen species, proteinase 3, cathepsin G, and elastase. Upon arrival to the site of the affected tissue, neutrophils are activated either through direct contact with the pathogens or stimulation by the cytokines secreted by the resident tissue cells. Upon activation, neutrophils phagocytose any available microbes and release the contents of their toxic granules into the phagosomes containing the invading organism. As one can imagine, the release of these highly potent effectors can cause significant collateral tissue damage in addition to killing the invading organisms, so damage to host tissue is inevitable.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


