Acquired Lesions of The Lung and Pleura
Empyema
Epidemiology
Although overall rates of bacterial pneumonia have been declining in children, the incidence of complications such as parapneumonic effusions and empyema has increased.1 In the USA, pneumonia in children occurs at an estimated rate of 30–40 per 100,000.2 Parapneumonic effusions (PPE) can complicate pediatric pneumonia in 28–53% of patients.3,4 In children less than two years of age, the incidence of empyema doubled over a 10-year span, increasing from 3.5/100,000 in 1996–1998 to 7/100,000 in 2005–2007.5 Similarly, in patients between 2 to 4 years age, empyema rates nearly tripled from 3.7/100,000 to 10.3/100,000 during the same time period. While empyema in children is less serious compared to adults where mortality can approach 20%, it still poses a considerable burden on hospitals and families.
Pathogenesis
The natural progression of parapneumonic pleural disease has been outlined with three to four stages of increasing complexity.6–8 The pre-collection stage involves pleuritis and inflammation. This is followed by the exudative stage, which is a simple PPE, and is characterized by clear, free-flowing pleural fluid with a low white cell count. The fibrinopurulent stage is a complicated PPE (empyema) marked by the deposition of fibrin and purulent material in the pleural space and an increase in the leukocyte count of the fluid. Septations and fibrin strands begin to develop. These result from decreased fibrinolytic activity allowing increased fibrin deposition. The result is a procoagulant environment that leads to the development of solid material in the form of septations followed by loculations of purulent fluid (Fig. 23-1).9 The most advanced state is termed the organized stage when a thick peel is established. This peel can entrap the lung and result in chronic restrictive lung disease. While these stages are outlined in sequential progression, there is no certainty that each stage will progress to the next. Although outlined as a simple progression, the degree of patient illness may not correspond with these stages, depending on the extent of the concomitant parenchymal disease or the inflammatory response to the infectious processes.
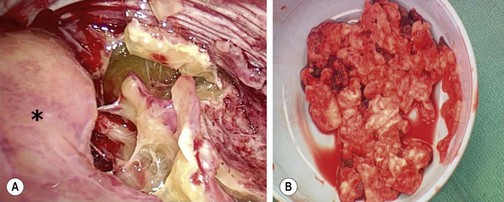
FIGURE 23-1 (A) The thoracoscopic view shows the inflammatory septations that can develop with empyema. The collapsed lung is marked with an asterisk. (B) Note the thick, solid purulent material that is often found in these patients.
As the stages advance, the chemistry of the parapneumonic fluid changes: glucose decreases, pH decreases, and lactate dehydrogenase (LDH) rises. The Light criteria for complicated PPE include pH <7.2, lactate dehydrogenase >1000 units, glucose <40 mg/dL or < 25% of the blood glucose, Gram stain or culture positive, and loculations or septations documented with imaging.10 Retrospective data suggests that a prolonged fever, a low pleural fluid pH and glucose along with a high LDH pleural/serum ratio are associated with more severe disease.11 Another study, using multivariate logistic analysis of a retrospective dataset, found that a pleural fluid pH less than 7.27 was the only significant factor for the formation of fibrin with/without septations.12 A third study suggested that a tumor necrosis factor level greater than 80 pg/mL in the pleural fluid suggests a complicated effusion.13
In a 1995 review, Light proposed a detailed classification which defines seven different stages from nonsignificant PPEs to a complex empyema. Recommendations on appropriate therapy were also described, which ranged from observation to thoracoscopic debridement/decortication (Table 23-1).14 A consensus statement from the American College of Chest Physicians in 2000 noted that more interventional therapy was needed as the stage of effusion progressed.15 Similarly, in another retrospective study, a pleural fluid pH less than 7.1 was found to result in a sixfold increase in the likelihood of operative intervention.16 While all these criteria may document the physiologic progression of disease, the clinical relevance of a patient’s pleural fluid analysis may not be that important because once symptoms develop and fluid with or without septations are found, intervention is needed.
TABLE 23-1
Classification Scheme for Parapneumonic Effusions and Empyema
| Categorization | Pleural Fluid Findings |
| Class 1 | |
| Nonsignificant parapneumonic effusion | Small <10 mm thick on decubitus X-ray |
| Class 2 | |
| Typical parapneumonic effusion | >10 mm thick Glucose >40 mg/dL, pH>7.20 Gram stain and culture negative |
| Class 3 | |
| Borderline complicated parapneumonic effusion | 7.00<pH<7.20 and/or LDH>1,000 and glucose >40 mg/dL Gram stain and culture negative |
| Class 4 | |
| Simple complicated parapneumonic effusion | pH<7.00 and/or glucose <40 mg/dL and/or Gram stain or culture positive Not loculated, no frank pus |
| Class 5 | |
| Complex complicated parapneumonic effusion | pH<7.00 and/or glucose <40 mg/dL and/or Gram stain or culture positive Multiloculated |
| Class 6 | |
| Simple empyema | Frank pus present Single locule or free flowing |
| Class 7 | |
| Complex empyema | Frank pus present Multiple locules |
Modified from Light RW. A new classification of parapneumonic effusions and empyema. Chest 1995;108:299–301.
Diagnosis
The diagnosis is usually a progressive clinical picture beginning with pneumonia. Patients with an empyema almost always demonstrate some degree of respiratory distress, malaise, persistent fever, or pleuritic chest pain.17–20 Diminished breath sounds with dullness to percussion on the affected side are found on examination. An ileus is common as is a lack of appetite.
Initial imaging is a chest radiograph (CXR) which shows poor penetration on the affected side. However, it is often difficult to distinguish between parenchymal consolidation and pleural fluid on a plain film.21 In a retrospective review of over 300 adult patients, CXR missed all effusions that were significant enough to warrant drainage when compared with subsequent computed tomography (CT) scans (Fig. 23-2A).22 Decubitus films may be helpful in distinguishing between nonloculated and loculated effusions.21
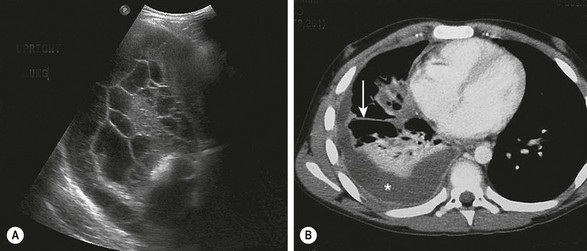
FIGURE 23-2 Ultrasonography and/or CT of the chest are helpful during the initial evaluation of children with a pleural effusion and possible empyema. (A) In the ultrasound study, note the loculations identified in the pleural fluid. (B) On the CT scan, a large pleural effusion (asterisk) is noted. Also there is collapse of the underlying lung parenchyma as well as septations (arrow).
Ultrasonography (US) is portable, relatively inexpensive, and does not involve radiation (Fig. 23-2B). It is very sensitive in diagnosing loculated fluid and can be used to guide percutaneous drainage and catheter placement.23,24 Some authors suggest that ultrasound is superior to CT in the identification of pleural debris or loculations.25,26 ultrasound can reliably differentiate between parenchymal and pleural based processes.6 A post hoc review of a prospective trial in children found that in 31 patients in whom both CT and ultrasound were performed, there was no advantage of CT over ultrasound in most cases.1 Two independent series reviewed the implementation of an algorithm using initial ultrasound in children with complicated pneumonia.27,28 Both demonstrated a significant reduction in hospitalization and a decrease in the use of CT without an increase in the rate of operative management or pleural drainage. A small retrospective review comparing ultrasound and CT found that CT had no advantage in most cases and suggested that CT should be used in complex cases only, such as patients undergoing operation or thought to have parenchymal abscesses or a bronchopleural fistula.26 CT has been found to be inferior to ultrasound at demonstrating fibrin strands or septations within the pleural fluid.25 Neither CT nor ultrasound is completely reliable in differentiating the specific stages of parapneumonic disease.1,25,26 The main disadvantages of ultrasound appear to be lack of 24-hour ultrasound availability and being operator dependent.
With pleural space disease, CT with intravenous contrast can differentiate between parenchymal and pleural processes, identifying pleural thickening and loculations.25 CT radiation exposure has raised the concern for a long-term cancer risk.29 Currently, chest CT scans can be performed effectively with the use of automatic dose modulation software that limit the radiation dose.25 Despite this ability to limit radiation exposure with CT scan, consensus statements are clear in their recommendations for performing CT only when needed, such as preoperative planning in some cases.6,7
Management
Parapneumonic Effusion
After the diagnosis of PPE is made, the first branch in the management algorithm depends on the nature of the fluid. With a free-flowing effusion and no solid components or signs of frank pus, the nature of the intervention will depend on the size of the effusion and the symptoms. Classifying the size is difficult to define precisely. However, in general, small effusions are defined as having 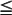 1 cm rim of fluid, moderate effusions have a 1–2 cm rim, and large effusions have
1 cm rim of fluid, moderate effusions have a 1–2 cm rim, and large effusions have 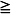 2 cm rim on decubitus films. A recently published twelve year retrospective study in children classified small effusions as less than a quarter hemithorax opacification, moderate effusions as a quarter to a half opacification, and large effusions as more than half opacification based on upright films.30 In this study, the authors found that small and most moderately sized effusions could be effectively managed without drainage and without an increase in the length of hospitalization or other complications. The authors suggest that interventions should be based on symptoms, not the size of the effusion alone.
2 cm rim on decubitus films. A recently published twelve year retrospective study in children classified small effusions as less than a quarter hemithorax opacification, moderate effusions as a quarter to a half opacification, and large effusions as more than half opacification based on upright films.30 In this study, the authors found that small and most moderately sized effusions could be effectively managed without drainage and without an increase in the length of hospitalization or other complications. The authors suggest that interventions should be based on symptoms, not the size of the effusion alone.
Symptoms leading to intervention generally are feeding intolerance, tachypnea, and an increasing oxygen requirement. A retrospective case series in children found respiratory distress on presentation was related to prolonged stay and a higher likelihood for intervention.31
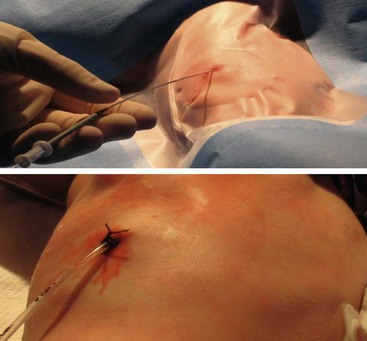
After deciding to drain the effusion, options include single or multiple thoracentesis versus tube thoracostomy or catheter drainage. A prospective, nonrandomized pediatric series compared treatment with repeated ultrasound-guided needle aspirations vs tube thoracostomy. There was a mean 2.4 drainages/patient in the aspiration group, but similar length of stay.32 While this approach may be reasonable in an older child who can tolerate the procedure with local anesthesia, it would likely not be appropriate in younger children. The British Thoracic Society guidelines recommend a chest tube for cases in which the first thoracentesis fails to adequately drain the effusion in order to avoid multiple thoracentesis attempts.6 A retrospective series compared 33 children who underwent chest tube placement on the basis of effusion size and/or thoracentesis fluid analysis vs 32 who were treated conservatively with tube thoracostomy only for progressive symptoms or mediastinal shift.33 The authors found no difference in duration of hospitalization and suggested frugal use of chest tubes.3 In a series of 405 adult patients, 266 had a chest tube smaller than 14 French compared to 139 with larger tubes.34 There was no difference in the ability to drain the effusion. Furthermore, smaller caliber tubes did not hinder the use of fibrinolytics. In a retrospective series of 20 children treated with standard chest tubes compared to 12 treated with pigtail tubes, no outcome differences were found.35 In patients with pleural effusions or empyema, we exclusively use 12 French Thal-Quick chest tubes (Cook Critical Care, Bloomington, Indiana, USA) that are inserted using the Seldinger technique (Fig. 23-3). We have not felt it necessary to use image guidance in the majority of patients.
Empyema
Empyema is diagnosed by identifying solid components in the pleural fluid on imaging studies or pus during thoracentesis or tube catheter placement. The definitive management for empyema has traditionally been operative debridement, which is currently performed via video-assisted thoracoscopy surgery (VATS).36–40 VATS has resulted in earlier and more complete resolution of empyema than chest tube drainage alone in both retrospective and prospective studies, translating into shorter hospitalization with VATS as the initial therapy.41–44 A retrospective series of 89 children undergoing primary thoracoscopic debridement/decortication found a 12% risk for needing another procedure to address ongoing disease or a complication.45 However, the superiority of operative mechanical debridement as a definitive management strategy has been increasingly challenged by chemical debridement with fibrinolysis.
Examples of fibrinolytics include urokinase, streptokinase, and tissue plasminogen activator (tPA). Since fibrin is a predominant component of the extracellular matrix upon which septations and solid debris develop, fibrinolysis has been shown to be superior to tube thoracostomy alone in retrospective and prospective studies.46–52 These studies include direct comparisons between the two treatment options as well as the use of fibrinolytic therapy after failure of tube thoracostomy.
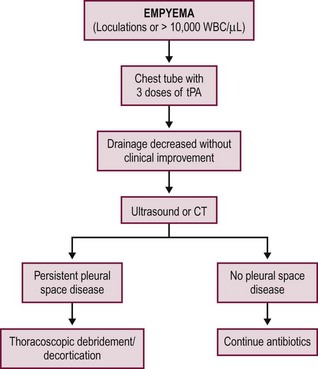
Two prospective, randomized trials have been conducted independently comparing fibrinolysis to VATS for empyema in children.53,54 The studies were conducted in the UK and USA in 60 and 36 patients, respectively. Both studies compared the instillation of three intrathoracic doses of fibrinolytic agents to thoracoscopy as the initial therapy for empyema. The first fibrinolytic dose in both studies was given at the time of diagnosis and/or chest tube insertion followed by two additional doses in 24-hour increments to complete the fibrinolytic course over a 48-hour period. The results were highly concordant as both studies found no difference in duration of hospitalization between the two interventions. The common parameters between the two studies are outlined in Table 23-2. The USA study found no difference in days of tube drainage, days of fever, doses of analgesics, or oxygen requirements.54 Both studies documented thoracoscopic debridement was more expensive and both utilized an intention-to-treat analysis so the length of stay and total charges included the patients who failed fibrinolysis and subsequently required VATS. The failure rate for fibrinolysis was 16.6% in both studies. This failure rate was similar to previous studies investigating the utility of fibrinolysis.48,55–59 An example of a first-line fibrinolytic approach is outlined Figure 23-4. A similar algorithm has been proposed based on a review of the literature.60
TABLE 23-2
Common Outcome Variables Reported between the Two Prospective Trials Comparing Fibrinolysis to VATS in Children

VATS, video-assisted thoracoscopic surgery; tPA, tissue plasminogen activator.
aCharges are in thousands of British pounds and thousands of ultrasound dollars.
One of the groups that performed the prospective randomized trial comparing fibrinolysis to thoracoscopic debridement recently reported their data using an evidence-based algorithm with primary fibrinolytic therapy. One hundred and two consecutive patients were treated with fibrinolysis following completion of the prospective randomized trial and 16 patients (15.7%) required subsequent thoracoscopic debridement and decortication. The length of hospitalization with fibrinolysis was 6.1 ± 2.5 days. In those patients that failed fibrinolytic therapy, the length of hospitalization after thoracoscopic debridement was 5.9 ± 3.7 days. Factors correlating with the need for thoracoscopy included age, gender, and initial drain output. However, none of these variables were independent predictors.61
Lung Abscess
Pulmonary abscess is often assumed to develop as a primary process in a previously normal lung, usually as a result of a necrotizing pneumonia. However, a pulmonary abscess in a child without an antecedent history ought to be considered as a secondary abscess which arises in an infected pulmonary anomaly such as a cystic pulmonary adenomatoid malformation, bronchogenic cyst, or foreign body. Most primary lung abscesses are located in the posterior segment of the right upper lobe and the superior segments of the right and left lower lobes (Fig. 23-5). In contrast, secondary and/or recurrent collections may be found in multiple locations with no specific anatomic predilection.
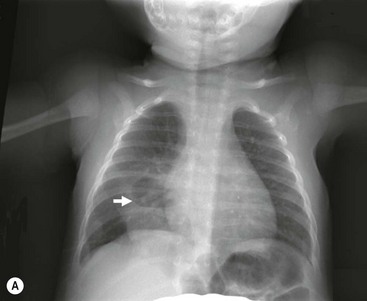
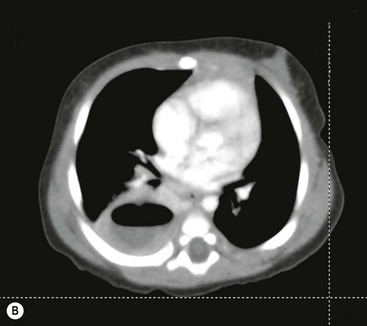
FIGURE 23-5 (A) This young infant was found to have this lung abscess in the right lower lobe on chest radiograph. Note the air-fluid level (arrow). (B) The lesion is seen on the CT as well. Workup did not reveal an associated congenital pulmonary airway malformation and she recovered with antibiotic therapy.
In the patient with known pneumonia, the non-responding patient who develops a suspicious lesion on chest film should undergo CT to evaluate for an abscess. The treatment of a lung abscess in an infant or child follows the same basic principles of postural drainage and pulmonary toilet used in adults, but it is more often ineffective, secondary to the small size of the airway.62,63
In general, an operation should be avoided as abscesses can usually be successfully treated with antibiotics alone.64,65 CT-guided drainage or catheter placement is usually needed if the lesion is peripheral and not connected to the airway, .66–68 Retrospective data suggests that drainage shortens hospitalization and facilitates earlier recovery.69 A recent series of 11 children with pulmonary abscess treated with thoracoscopic drainage fared well without complications which may be a good option when less invasive maneuvers fail.70 Alternatively, pulmonary resection may be required for abscesses that are more centrally located and resistant to medical management.62,71 Patients with fungal isolates and immunocompromised patients generally require early and aggressive pulmonary resection. However, as a general statement, patience should be employed in managing pulmonary abscesses.