Fig. 3.1
Partial placental abruption
3.2 Epidemiology
The prevalence of placental abruption (PA) ranges from 0.4 to 1 % of all pregnancies and depends on the population. In Nordic countries, approximately 0.38–0.51 % of all pregnancies are complicated by placental abruption. This rate tends to be higher in the USA (0.6–1 %). In the developing areas, e.g., in some African countries, PA incidence can reach up to 2 %. According to epidemiological reports, the incidence of PA has been steadily increasing. The recurrence rate of placental abruption is 8.8 and 25 % after one and two events, respectively [1–8, 11].
Preterm detachment of the placenta is potentially disastrous, especially for the fetus, with perinatal mortality of 25 %. The incidence of PA is the highest at 24–26 weeks of gestation and decreases with advancing gestation. Over 50 % of the cases occur before 37 completed weeks of gestation. In the developed countries, almost 10 % of preterm deliveries are caused by placental abruption [9, 10]. High neonatal mortality is mainly related to prematurity, low birth weight, IUGR, and asphyxia. A 25-fold higher mortality is present even in term pregnancies complicated by PA. Although the stillbirth rate due to abruption has declined, it remains an important cause of neurological deficits in the first year of life. Approximately 20 % of the survivors delivered between 26 and 36 weeks present cerebral palsy [12].
Maternal morbidity and mortality associated with PA include massive blood loss, disseminated intravascular coagulation, emergency hysterectomy, the need for blood transfusion, renal failure, and maternal death. In Western Europe and the USA, PA-related maternal mortality is 0.4 per 1,000 births. The rate of maternal deaths depends on the level of medical care, ranging from 1 to 4.7 in the developing countries [10, 13, 14].
3.3 Pathology
The underlying pathomechanism of placental abruption remains unclear. PA seems to be a multifactorial disease, with different causative patterns in preterm and term gestations. Several risk factors, i.e., abdominal trauma, hypertension, or coagulopathy, have been defined as strongly related to placental abruption. However, there are still many cases which occur without any perceptible causes [15].
The placenta is fixed to the uterine wall by the anchoring villi. When spiral arteries lack the physiologic trophoblast invasion, abruption might occur. Infusion of thromboplastic material induces disseminated intravascular coagulation. Hypertonicity of the uterus occurs probably to prevent the entrance of further thromboplastic material into the maternal circulation. The conditions which contribute to the avulsion of the anchoring placental villi from the expanding lower uterine segment, which in turn leads to bleeding into the decidua basalis, can be explained by a number of hypotheses [16–20].
3.3.1 Pathway of Acute Inflammation
Infection (chorioamnionitis) and tissue injury (trauma, PROM) cause a rapid release of macrophage activators (lipopolysaccharide, heat shock protein 60) at the maternal-fetal interface. Increased production of pro-inflammatory cytokines such as tumor necrosis factor-α and interleukin-β leads to an elevated release of matrix metalloproteinases by trophoblast cells. In consequence, increased level of necrosis and apoptosis processes leads to preterm placental detachment (Fig. 3.2).


Fig. 3.2
Pathway of acute inflammation. Definition: PROM premature rapture of membranes, LPS lipopolysaccride, Hsp heat shock protein, IL interleukin, TNF tumor necrosis factor, MMP matrix metalloproteinase
3.3.2 Pathway of Chronic Processes
A strong coincidence between PA and other pregnancy complications, e.g., preeclampsia, preterm labor, SGA, or diabetes, can indicate an impaired placentation process in early pregnancy as the main pathological mechanism. Long-standing vascular lesions lead to elevated oxidative stress and platelet activation, resulting in chronic inflammatory processes. Chronic inflammation pathway is more common for premature PA (Fig. 3.3).


Fig. 3.3
Pathway of chronic process. Definitions: PE preeclampsia, SGA small for gestational age, GDM gestational diabetes mellitus, PAF platelet activation factor
3.3.2.1 Fetal-to-Maternal Hemorrhage
In cases of nontraumatic placental abruption, the detachment occurs in the layer of maternal decidua. The origin of the bleeding is almost always maternal. The evidence for fetal-to-maternal bleeding has been reported in only 20 % of the cases, but with the volume of ≤10 mL [22]. The occurrence of fetal bleeding is much more likely in case of preceding trauma, when placental abruption follows a tear or fracture of the placental tissue, resulting in fetal bleeding [21].
3.4 Risk Factors
Lack of one hypothesis about the mechanism which leads to placental abruption is the reason why identification of the most frequent risk factors for this severe pregnancy complication is a subject of much heated debate. The literature offers reports on numerous multicenter analyses which have been conducted to identify the most common risk factors (Table 3.1) [38].
Table 3.1
Risk factors for placental abruption
Risk factor | Odds ratio |
|---|---|
Maternal risk factors | |
Chronic hypertension | 1.8–2.4 |
Hyperhomocysteinemia | 1.8–5.3 |
Thrombophilia | 1.4–7.7 |
Uterine anomaly | 8.1 |
Historical risk factors | |
Cesarean section | 1.3–2.4 |
Miscarriages | 1.4–3.4 |
Placental abruption | 3.2–25.8 |
Preeclampsia | 1.9 |
Stillbirth | 1.6–13.1 |
Behavioral risk factors | |
Cigarette smoking | 1.5–2.5 |
Alcohol use | 1.6–2.8 |
Cocaine use | 3.9–8.6 |
Pregnancy-associated risk factors | |
Pregnancy-induced hypertension | 1.5–2.5 |
Preeclampsia | 1.9–4.4 |
PROM | 1.8–5.9 |
Chorioamnionitis | 2.5–3.3 |
Placenta previa | 3.2–5.7 |
Multiple pregnancy | 2.0–2.9 |
Advanced maternal age (>35 years) and parity have the strongest correlation with placental abruption out of the sociodemographic and behavioral risk factors. Some authors also mention maternal age of <20. In general analyses, black ethnic origin, marital status (unmarried), and lower socioeconomic status are related to higher prevalence of placental abruption [24, 25].
Several studies have demonstrated that maternal smoking during pregnancy increases the risk for abruption even by 2.5-fold. Interestingly, paternal smoking doubles the risk. Probably, quitting smoking before pregnancy reduces the risk to the level of nonsmokers. Among drug-using women, cocaine use is the strongest risk factor for abruption, increasing the risk even by 8.6-fold [5, 23, 25, 26].
Among pregnancy-related risk factors, pregnancy-induced hypertension and preeclampsia have been demonstrated to have the strongest correlation with PA (2.5 and 4.4, respectively). Many studies have shown that the more severe the hypertension, the higher the risk for abruption [2, 6, 28, 29]. Other pregnancy complications which can increase the incidence of placental abruption include placenta previa, vaginal bleeding in the early pregnancy, and multiple gestations. The risk for PA in case of premature rupture of membranes is 5.9 % and is related to sudden changes of intrauterine pressure and increased risk for chorioamnionitis [1, 2, 6, 29].
Chronic hypertension is one of the most frequent maternal risk factors for PA. Chronic hypertension complicates 0.3–0.8 % of all pregnancies and often corresponds with other risk factors, including advanced maternal age, black race, smoking, and parity. According to the literature, chronic hypertension increases the risk for abruption by 2.4-fold [2, 27, 28]. Also, thrombophilia and hyperhomocysteinemia are strongly related with the risk for PA. Hyperhomocysteinemia is associated with folate and vitamin B12 deficiency, which can be the direct cause of the abruption. In most studies, homozygous methylenetetrahydrofolate reductase point mutation 677 is related to an even sevenfold increase of PA. Literature data about the wide range of thrombophilia are insufficient, but a Swedish study of Prochazka failed to show a correlation between factor V Leiden carrier rate and placental abruption [30–33].
In the group of history-related risk factors, special attention should be paid to patients after previous cesarean section. According to the literature, the first cesarean delivery increases the risk for abruption by 30–40 % in the next pregnancy, as compared to the first vaginal delivery. The risk increases to 52 % if the inter-pregnancy interval is <1 year [4, 34, 35].
Approximately 6 % of all trauma cases in pregnancy and 20–25 % of major traumas are associated with PA. The first manifestation of placental abruption after abdominal trauma occurs mainly within 6–48 h. In rare cases, placental abruption can manifest even up to 5 days after the initial trauma [21, 36, 37].
3.5 Clinical Findings
3.5.1 Vaginal Bleeding
Placental abruption is the leading cause of vaginal bleeding in the second part of pregnancy. Vaginal blood loss is presented in approximately 80 % of all cases. Hemorrhage into the decidua basalis occurs as the placenta separates from the uterus, resulting in external bleeding or formation of a hematoma behind the placenta. Further bleeding accelerates the detachment of the placenta from the uterine wall, causing vessel compression and compromising blood supply to the fetus. This alarming sequence of events may lead at the end stage to myometrial rupture. The amount of vaginal bleeding can vary greatly, and it does not necessarily correspond to how much of the placenta has separated from the inner wall of the uterus (Fig. 3.4b) [39].


Fig. 3.4
Placental abruption with concealed hemorrhage (a) and vaginal bleeding (b)
In some cases of central placental abruption, blood does not escape externally but remains within the retroplacental area. Concealed hemorrhage soon leads to a complete detachment of the placenta. It is related to higher rate of fetal death and maternal consumptive coagulopathy. No visible vaginal bleeding in case of severe placental abruption is a rather poor prognostic sign, and, in most cases, the diagnosis is typically delayed (Fig. 3.4a).
3.5.2 Abdominal Pain
Abdominal pain often occurs suddenly and is mostly defined as strong and sharp. Pain may be limited to the area where placental detachment begins or may give a general tenderness in the abdomen. It may radiate to the back in cases when the placenta is localized on the posterior wall of the uterus. Pain is often followed by nausea and vomiting.
3.5.3 Contractions
During the examination, the uterus may be disproportionately enlarged and an increased tonicity is often present. Moreover, rapid, painful contractions which follow one another can occur. In case of massive placental abruption, the uterus becomes hard and very painful.
3.5.4 Fetal Distress
Placental abruption results in fetal distress in almost 60 % of the cases [39]. Altered fetal activity is one of the most alarming signs. The patient may present with decreased or absent fetal movements. Cardiotocography (CTG) reveals symptoms of fetal distress, which occurs mainly due to placental separation, maternal hemorrhage, fetal hemorrhage, or uterus hypertonus. In case of severe placental abruption, no fetal heart tone may be demonstrated (Fig. 3.5a, b). Rarely, intrauterine fetal death is the only sign of PA, and the diagnosis is made postpartum, after visualization of the blood clot on the placental surface.


Fig. 3.5
Signs of fetal distress due to placental abruption in the third trimester of pregnancy in cardiotocography. Upper panel: the fetal hart rate demonstrates late decelerations (a) and baseline bradycardia (b)
3.6 Classification
Depending on the extent of the separation, placental abruption can be partial or complete. Due to localization of the detachment, abruption can be classified as marginal or central.
Clinical classification contains 0–3 classes and is based on the severity of the clinical symptoms.
Class 0 – asymptomatic:
The diagnosis is made retrospectively by finding an organized blood clot or a depressed area on a delivered placenta.
Class 1 – mild (48 % of the cases); the characteristics include the following:
No vaginal bleeding to mild vaginal bleeding
Slightly tender uterus
Normal maternal BP and heart rate
No coagulopathy
No fetal distress
Class 2 – moderate (27 % of all cases); the characteristics include the following:
No vaginal bleeding to moderate vaginal bleeding
Moderate to severe uterine tenderness with possible tetanic contractions
Maternal tachycardia with orthostatic changes in BP and heart rate
Fetal distress
Class 3 – severe (28 % of all cases); the characteristics include the following:
No vaginal bleeding to heavy vaginal bleeding
Very painful tetanic uterus
Maternal shock
Hypofibrinogenemia (i.e., <150 mg/dL)
Coagulopathy
Fetal death
3.7 Diagnosis
Placental abruption should be taken into consideration in each case of vaginal bleeding in the second and third trimester of pregnancy. The presence of a large retroplacental hematoma in most cases results in typical signs such as abdominal pain, contractions, and uterine tenderness. In case of strong suspicion of PA and signs of fetal distress, immediate management should be initiated, without any delay caused by an additional examination. Regardless, numerous hematomas do not reach significant size and remain asymptomatic. If the course of the events is not rapid, an ultrasound examination could be performed. According to the literature, sensitivity of ultrasound diagnosis of a hematoma is not higher than 50 %. Nevertheless, due to its availability and short time required for the exam, ultrasound examination of the placenta is considered very helpful and can be useful in the differential diagnosis between PA and placenta previa (Fig. 3.6a, b).


Fig. 3.6
Transvaginal sonogram of total placenta previa (a) and marginal placenta previa (b)
Placental abruption results in a wide variety of sonographic findings, e.g., preplacental fluid collection (between the placenta and the amniotic fluid); jellylike movements of the chorionic plate induced by fetal activity; presence of marginal, subchorionic, or intra-amniotic hematoma; and increased heterogeneous placental thickness (>5 cm in the perpendicular plane) (Figs. 3.7, 3.8, 3.9, and 3.10). Most of them depend on the location of the bleeding and its duration. The locations of the hemorrhage can be categorized as follows: most frequent, subchorionic (between the myometrium and the placental membranes), retroplacental (between the placenta and the myometrium), and preplacental (between the myometrium and the amniotic fluid). Most subchorionic hematomas are contiguous with the placental margin. However, in some cases the majority of the blood separates from the placenta and forms a hematoma on the myometrial surface, opposite the placenta.





Fig. 3.7
Transvaginal presentation of acute subchorionic hematoma (white arrows) in early pregnancy

Fig. 3.8
Transabdominal scan. Presence of marginal hematoma in patient with the history of slight vaginal bleeding

Fig. 3.9
Transabdominal sonogram of subchorionic hematoma (white arrows)

Fig. 3.10
Transabdominal sonogram of marginal sonolucent hematoma (a–c)
Echogenicity of a hematoma strictly correlates to the time of the bleeding. Acute bleeding (<48 h) can be presented as a hyperechoic area, defined as equal to or greater than the adjacent placenta. Acute hematoma often imitates placental tissue, making the correct diagnosis challenging even for experienced sonographers (Fig. 3.11a, b). In order to avoid misdiagnosis, the examination may need to be repeated in the next 24 h. In the next 3–7 days, the hematoma is presented as a hypoechogenic area similar to the myometrium. After 2 weeks, the major part of the hematoma becomes sonolucent (anechoic) and can be compared to the amniotic fluid.
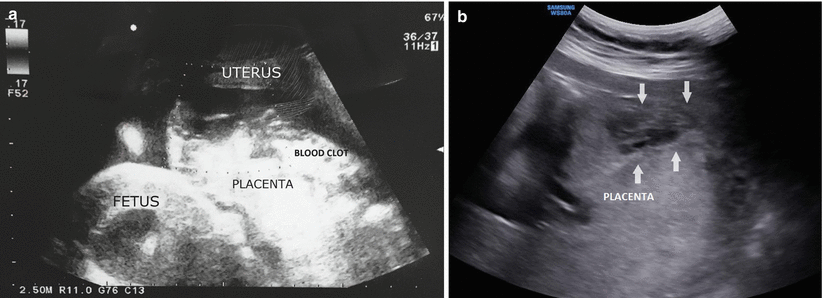

Fig. 3.11
Acute placental abruption. Transabdominal sonogram of a patient at 34 weeks’ gestation with sudden abdominal pain, signs of fetal distress, and no vaginal bleeding (a). Transabdominal sonogram of a patient at 32 weeks’ gestation with chronic hypertension (b). Emergency cesarean delivery confirmed acute placental detachment in both patients
The volume of the hematoma and the size of the detached area of the placenta are the most appropriate prognostic factors in a sonographic examination. Three perpendicular diameters (D) need to be measured and put into the following formula:0.52 × (D1 × D2 × D)3, to estimate the volume of the hemorrhage (Figs. 3.12 and 3.13). In cases when the detachment exceeds 50 % of the placenta or the hematoma volume is over 50 ml, the prognosis is very poor [40, 42–44].



Fig. 3.12
Chronic placental abruption. Transabdominal scan. Presence of subchorionic hypoechogenic hematoma

Fig. 3.13
Transabdominal sonogram. Measurement of the volume of hematoma and the size of the detachment in the third trimester of pregnancy
Color Doppler flow images add a considerable value to sonographic examination by excluding, i.e., placental vascular abnormalities, adhesive disorder, or vasa previa (Fig. 3.14).


Fig. 3.14
Transvaginal presentation with color Doppler of placenta previa. Sonogram of a 35-year-old patient in 26 weeks of gestation presenting moderate vaginal bleeding
Importantly, lack of sonographic confirmation does not exclude placental abruption and should never delay management .
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


