Abnormalities of the Lungs
M. Grisel Galarza and Ilene R. S. Sosenko
SIGNS OF RESPIRATORY DISTRESS
Respiratory distress syndrome in the newborn (discussed in detail in Chapter 54) is the most frequent problem that results in neonatal intensive care unit admission. It is essential to recognize, evaluate, and differentiate between the many possible causes of respiratory distress, including those that do not primarily involve the lung. The presenting symptoms and severity of these symptoms may alert the physician in a life-threatening situation.
One or more of the following may characterize respiratory distress in the newborn: tachypnea, grunting, nasal flaring, and chest retractions. A newborn normally breathes at a rate of 30 to 60 breaths per minute. A newborn with tachypnea may breathe at a faster rate to maintain adequate ventilation and may attempt to decrease airway resistance by flaring the nares. The infant may try to maintain lung volume by partially closing the glottis during expiration, thereby producing grunting sounds. Chest retractions may occur with parenchymal lung disease or an obstructed airway. Additional signs such as cyanosis, gasping, apnea, stridor, or choking should alert the physician of a more severe respiratory problem. Respiratory distress in the newborn is a common presentation for a wide variety of disorders shown in Table 50-1.
TRANSIENT TACHYPNEA
Transient tachypnea of the newborn (TTN) is a self-limited, usually benign disease, affecting the term infant and late-preterm infant soon after birth. Avery and coworkers described the clinical and radiographic features of this condition in 1966 and attributed it to a delayed absorption of fetal lung fluid.1 More recently, Bland2,3 described TTN as a persistent postnatal pulmonary edema, since some of the fluid may enter the lungs from the pulmonary circulation postnatally. TTN occurs in approximately 11 infants per 1000 live births and is more common in males. It is associated with cesarean section delivery,4 the use of maternal labor analgesia or anesthesia, gestational diabetes, and perinatal asphyxia.
 PATHOGENESIS
PATHOGENESIS
Fetal lung fluid is essential for normal lung development, is a secretion intrinsic to the lung, and is not aspirated amniotic fluid.5 Fetal lung fluid is the result of active chloride secretion by the distal lung epithelia.6-8 The chloride ion draws fluid from the pulmonary circulation into the airways of the lung, where this fluid maintains expansion of the potential air spaces and allows for normal fetal lung growth and development.3,8
A few days before labor begins, secretion of lung fluid decreases in preparation for air breathing. The notion that during a vaginal delivery lung fluid is “squeezed” out accounts for only a fraction of the fluid cleared. The process of fetal lung fluid absorption is much more complicated, and labor plays an essential role.3 From the onset of labor to a few hours after birth, a large amount of extravascular lung fluid is actively absorbed by the lung epithelium. This occurs when the epithelium switches from predominant chloride secretion to predominant sodium absorption. Active transport of sodium across the lung epithelium via sodium channels at the apical membranes and sodium potassium exchange (Na+/K+-ATPase) at the basolateral membranes of epithelial cells drive the fluid from the lung lumen to the interstitium, with subsequent absorption into the vasculature and clearance by lymphatics (Fig. 50-1).8 In transgenic mice, the absence of functional epithelial sodium channels causes early neonatal death secondary to respiratory failure.9 The pulmonary expression and function of these sodium channels increase as birth approaches. With the onset of labor, the changes in ion transport by the fetal lung epithelium are “switched on” by catecholamines and other hormone surges and possibly maintained “on” by increased oxygenation after birth.8,10
Table 50-1. Causes of Respiratory Distress in the Newborn Infant
Respiratory Diseases |
Obstructive |
|
Nasal |
Choanal |
Laryngeal |
Tracheobronchial |
|
|
|
|
Chest wall abnormalities/diaphragmatic hernia |
Malformations of the mediastinum and lung parenchyma |
|
|
|
|
|
|
Lung parenchymal and vascular diseases |
|
|
|
|
|
|
|
Cardiac Diseases |
Cyanotic |
|
|
|
|
|
Acyanotic |
|
|
|
|
Neurological Disorders |
Trauma |
Intraventricular hemorrhage |
Hypoxic ischemic encephalopathy |
Seizure disorder |
Meningitis |
Other Disease Processes |
Sepsis |
Polycythemia |
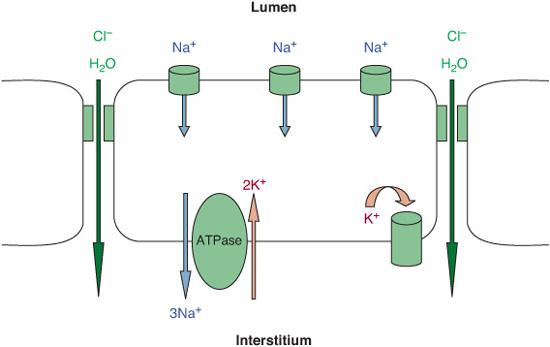
FIGURE 50-1. Model of fetal lung fluid absorption by epithelial cells. Fluid absorption results from vectorial transport of Na+, driven by Na+/K+-ATPase. The resultant electrochemically increased gradient leads to passive Na+ absorption via apical Na+-permeant channels that is extruded by Na+/K+-ATPase out of the cell. Cl and water passively follow the Na+ ions through paracellular or intracellular pathway.
Other factors that may contribute to the clearance of fetal lung fluid include the osmotic pressure gradient due to the lower protein content of lung fluid versus plasma and lung inflation at birth that increases transpulmonary pressure. Elevated left atrial pressure may inhibit lung fluid absorption. Under normal conditions, the physiologic process of fetal lung fluid clearance begins before birth and continues for 4 to 6 hours at birth. This process may be adversely affected by preterm delivery, delivery without labor, conditions in which left heart failure or hypoxia cause elevated pulmonary vascular pressure, and low plasma protein concentration.
 CLINICAL FEATURES AND DIAGNOSIS
CLINICAL FEATURES AND DIAGNOSIS
The infant with transient tachypnea of the newborn (TTN) presents with tachypnea shortly after birth and mild to moderate signs of respiratory distress, such as nasal flaring, subcostal and intercostal retractions, and grunting during expiration. These infants have mild cyanosis and often require oxygen therapy but rarely need mechanical ventilation. On physical examination, diffuse crackles and rhonchi during the first few hours after birth result from residual fluid within the air spaces of the lungs. Blood gas analysis may be normal or may reveal mild alkalosis and hypoxemia. The characteristic radiographic findings are prominent pulmonary vascular markings especially around the hila, diffuse parenchymal infiltrates, widened interlobar fissures, and some degree of hyperinflation with flattening of the diaphragm. Pleural effusions may also be present, and the cardiac silhouette may appear enlarged (Fig. 50-2).
A diagnosis of TTN in a newborn infant with respiratory distress is made after other lung and cardiac disease have been excluded. Follow-up radiographs assist in differentiating TTN from other lung diseases such as pneumonia, meconium aspiration syndrome, and hyaline membrane disease, where abnormal radiographic findings may persist. Differential diagnoses also include air leaks, airway obstruction, diaphragmatic hernia, lung hypoplasia, and congestive heart failure, which can be excluded by chest radiograph and clinical progression.
 MANAGEMENT AND OUTCOME
MANAGEMENT AND OUTCOME
Supportive care and supplemental oxygen are all that may be required for infants with uncomplicated TTN. The need for mechanical ventilation or a prolonged course of oxygen is rare with TTN and may indicate another lung process. Most of these infants are also evaluated for sepsis, and empiric antibiotic therapy is given for 48 to 72 hours while awaiting blood culture results, clinical improvement, and radiographic evolution. The use of diuretics does not change the clinical course and may cause electrolyte problems. Infants with more moderate disease may respond well to nasal continuous positive airway pressure, which may increase the functional residual capacity and help clear lung fluid. For infants with uncomplicated disease, clinical improvement within 2 to 4 days is typical and prognosis is good without long-term pulmonary sequelae.
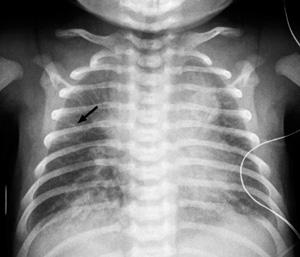
FIGURE 50-2. Anteroposterior radiograph of term newborn on day one with transient tachypnea shows bilateral linear opacities (arrow) extending from lung hila to peripheral lung fields.
CONGENITAL PNEUMONIA
Congenital pneumonia is acquired from the mother and presents with clinical signs at or shortly after birth. It may occur in the setting of maternal infection (chorioamnionitis) that may or may not be symptomatic in the mother. The estimated incidence of this early-onset pneumonia ranges from 5 to 50 per 1000 live births, with higher rates occurring in preterm and low-birth-weight infants and in the presence of maternal chorioamnionitis.11 It is a difficult disease to identify prospectively, with nonspecific clinical manifestations that are often shared with noninfectious diseases such as transient tachypnea of the newborn, meconium aspiration, and hyaline membrane disease. (See Chapter 230.)
 ETIOLOGY
ETIOLOGY
Transmission of the infectious organism from the mother may occur via 3 possible routes.12 The first is via hematogenous transmission whereby the infectious organism is acquired from a maternal bloodstream infection, crossing into the fetal circulation, invading the lungs or causing a more disseminated infection. The second means of transmission is via ascending infection in which the infectious organism is believed to originate in the vagina and then ascend the birth canal and spread to the fetal membranes. Ascending transmission of organisms may be increased by amniocentesis, repeated pelvic examinations, and placement of intrauterine catheters, and it may occur with or without rupture of amniotic membranes. Finally, infectious organisms may be transmitted by aspiration, which is believed to occur in utero when the fetus develops gasping after an asphyxial event and aspirates amniotic fluid containing infectious organisms.13 Early-onset pneumonia may also occur secondary to intrapartum acquisition of infectious organisms during the passage of the newborn through the birth canal. Many pathogens may lead to neonatal pneumonia, but the most common are those also identified in cases of early-onset neonatal sepsis. The usual organisms include group B Streptococcus, Escherichia coli, Listeria, and sometimes Candida albicans or herpes simplex virus.12,13
 CLINICAL FEATURES AND DIAGNOSIS
CLINICAL FEATURES AND DIAGNOSIS
The diagnosis of congenital pneumonia is based on a combination of historical, physical, and radiographic findings. Prenatal risk factors associated with congenital pneumonia in the newborn are the same as those associated with neonatal sepsis, including maternal fever and uterine tenderness, rupture of membranes for longer than 18 hours, foul-smelling amniotic fluid, maternal history of recurrent or recent untreated urinary tract infection, and previous infant with neonatal infection. Peripartum signs that may be associated with congenital pneumonia include fetal tachycardia, loss of beat-to-beat variability, meconium-stained amniotic fluid, and unexplained premature labor.
The clinical presentation of congenital pneumonia in the newborn is nonspecific, making the diagnosis difficult. It should be suspected in any newborn presenting with respiratory distress at or soon after birth and may include clinical signs that overlap with neonatal sepsis, such as temperature instability, hypoglycemia, hyperglycemia, lethargy, abdominal distention, and poor feeding. Some infants with congenital pneumonia may progress to septic shock and disseminated intravascular coagulation and develop pulmonary hemorrhage, air leaks, effusions, and pulmonary hypertension.
Radiographic and laboratory data are also nonspecific and share findings not only with neonatal sepsis but also with noninfectious processes such as transient tachypnea of the newborn, hyaline membrane disease, and meconium aspiration syndrome (eFig. 50.1  ). Blood cultures usually do not yield a pathogen (especially if maternal antimicrobial treatment was given during labor) but should always be obtained. Spinal fluid culture for the evaluation of congenital pneumonia is controversial because the yield is very low (< 1%), and a spinal tap may place a newborn with significant respiratory distress at risk. Culture and Gram stain of tracheal aspirate from a newly placed (within 8 hours of delivery) endotracheal tube may provide an early and specific diagnosis in some infants with congenital pulmonary infection.13 The use of inflammatory markers to support a diagnosis of infection, including pneumonia, is presently controversial.
). Blood cultures usually do not yield a pathogen (especially if maternal antimicrobial treatment was given during labor) but should always be obtained. Spinal fluid culture for the evaluation of congenital pneumonia is controversial because the yield is very low (< 1%), and a spinal tap may place a newborn with significant respiratory distress at risk. Culture and Gram stain of tracheal aspirate from a newly placed (within 8 hours of delivery) endotracheal tube may provide an early and specific diagnosis in some infants with congenital pulmonary infection.13 The use of inflammatory markers to support a diagnosis of infection, including pneumonia, is presently controversial.
 THERAPY AND PROGNOSIS
THERAPY AND PROGNOSIS
Supportive care should be provided, with a normothermic environment, adequate dextrose and fluid infusion, prompt correction of metabolic acidosis, supplemental oxygen, and mechanical ventilation if needed to maintain adequate oxygenation and ventilation. Antibiotic treatment with a broad-spectrum penicillin and an aminoglycoside is indicated until cultures are reported, and in many cases, it is continued for 7 to 10 days if a clinical diagnosis of congenital pneumonia is highly suspected even when cultures remain negative.
Prognosis is good for term infants with mild to moderate disease who have prompt treatment and no complications. Those infants with severe symptomatology and those who develop complications may have an increased risk of chronic lung disease, reactive airway disease, and childhood otitis media.
PULMONARY HEMORRHAGE
Massive pulmonary hemorrhage in the newborn infant is a catastrophic event that usually occurs between the second and fourth day of life. The incidence of pulmonary hemorrhage occurs at a rate of 1 to 12 per 1000 live births.14 Surprisingly, pulmonary hemorrhage was the principal cause of death in approximately 9% of neonatal autopsies.15 It occurs most commonly in preterm infants, usually associated with a patent ductus arteriosus.16 Other risk factors are perinatal conditions: preeclampsia, erythroblastosis fetalis, breech delivery, and maternal cocaine use; and neonatal factors: coagulopathies, infection, asphyxia, hypothermia, congenital heart disease, and aspiration.
 PATHOGENESIS
PATHOGENESIS
The pathophysiology of pulmonary hemorrhage in newborn infants is still not clear. Cole and colleagues17 found that the fluid from the lungs of infants with pulmonary hemorrhage was a plasma filtrate with low hematocrit, characteristic of hemorrhagic edema. The most important precipitating factor was postulated to be acute left ventricular failure from hypoxia and severe acidosis, causing increased pressure in the pulmonary microcirculation and increased filtration of fluid into the lung. Alveolar overdistension and high pulmonary capillary pressures cause breaks in the epithelial-endothelial barrier leading to leakage of hemorrhagic edema fluid into the air spaces.14 Toxic products from sepsis increase microvascular permeability in the pulmonary circulation, which may contribute to pulmonary hemorrhage. Preterm infants with increased cord blood neutrophil chemotaxis and chemiluminescence were reported to be at high risk of pulmonary hemorrhage, possibly related to entrapment of neutrophils in the pulmonary microcirculation and injury to the alveolar-capillary barrier.18 In the preterm infant with a large patent ductus arteriosus, pulmonary hemorrhage may result from injury to the microvasculature from high pressure and high flow.16,19 Surfactant therapy has also been associated with pulmonary hemorrhage, perhaps from the rapid increase in pulmonary blood flow that occurs with the improvement in lung function after surfactant administration.20
 CLINICAL FEATURES AND DIAGNOSIS
CLINICAL FEATURES AND DIAGNOSIS
The infant may present with increased respiratory distress, apnea, pallor, peripheral vasoconstriction, and bradycardia minutes before the onset of hemorrhage. Severe metabolic acidosis may precede the onset of hemorrhage. With massive hemorrhage, blood may be noted from the nose and mouth or from the endotracheal tube if the infant is intubated. The fluid may have a normal to low hematocrit. Chest radiographic findings are varied and nonspecific and may range from patchy infiltrates to complete opacification of the lung fields.
 MANAGEMENT
MANAGEMENT
A large pulmonary hemorrhage is a life-threatening event that requires immediate support and resuscitative measures. The airway must be cleared by suction to allow for adequate ventilation and oxygenation. Mechanical ventilation should provide sufficient mean airway pressure to maintain lung volume and sustain gas exchange. In a massive pulmonary hemorrhage, transfusion with packed red blood cells may be necessary to maintain adequate perfusion and blood volume. Caution should be used with excessive transfusion volumes which can increase left atrial pressure and cause pulmonary edema. Treatment of the underlying factor, such as sepsis, asphyxia, coagulopathy, or patent ductus arteriosus, should be undertaken promptly.
New treatment modalities being investigated include hemocoagulase and activated recombinant factor VII.15,21
 OUTCOMES
OUTCOMES
Mortality rates range from 30% to 60% in premature infants; approximately 60% of the survivors develop chronic lung disease.15,21 Mortality in term infants with pulmonary hemorrhage is related to the underlying precipitating disease process.
MECONIUM ASPIRATION SYNDROME
Meconium is present in the fetal intestine as early as 14 to 16 weeks of gestation. It is an odorless substance composed primarily of water and squamous cells, vernix, lanugo, blood, amniotic fluid, and intestinal secretions containing bile that imparts a black-green color. Passage of meconium in utero is rare before 32 weeks of gestation, making meconium aspiration syndrome (MAS) a disorder affecting near-term, term, and postterm infants.22-25
 INCIDENCE
INCIDENCE
Meconium staining of amniotic fluid occurs in approximately 10% to 15% of all live births, with 4% to 5% of these infants developing MAS.24 Respiratory failure is usually associated with the aspiration of thick or particulate meconium but may also occur in newborns in whom no meconium was recovered from below the vocal cords. Abnormalities of fetal heart tracings and low Apgar scores have been associated with the presence of meconium-stained fluid and MAS. Advanced gestation of 42 weeks or more increases the risk of meconium passage (30–50% of postterm infants), putting these infants at higher risk of MAS compared to term infants (7–22%) and preterm infants (< 2%).22,24
 PATHOGENESIS
PATHOGENESIS
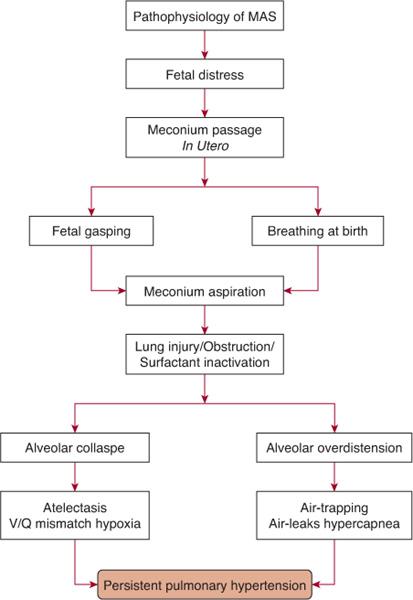
The passage of meconium in utero may be a response to fetal distress secondary to hypoxia, acidosis, or infection, which may cause relaxation of the anal sphincter and promote intestinal peristalsis.  Fetal distress may cause the passage of meconium in utero; if the distress is ongoing and leads to fetal hypoxia, gasping may occur, explaining the aspiration prior to delivery. Stillborn infants and infants who die soon after birth can have lungs full of aspirated meconium. Aspiration of meconium may also occur during and soon after delivery. Meconium may be present in the infant’s mouth and upper airway and may be aspirated with the onset of breathing.
Fetal distress may cause the passage of meconium in utero; if the distress is ongoing and leads to fetal hypoxia, gasping may occur, explaining the aspiration prior to delivery. Stillborn infants and infants who die soon after birth can have lungs full of aspirated meconium. Aspiration of meconium may also occur during and soon after delivery. Meconium may be present in the infant’s mouth and upper airway and may be aspirated with the onset of breathing.
Aspiration of meconium-containing amniotic fluid can cause lung injury via a number of mechanisms. The meconium initially will partially obstruct the airways with air trapping and overdistension of the lungs, which may lead to air leaks. It may cause complete obstruction of some airways, atelectasis, and ventilation-perfusion mismatch. Meconium may induce inflammation and a chemical pneumonitis, and it may also inactivate surfactant. The most severe respiratory disease occurs in infants with aspiration of thick-particulate meconium, but respiratory comprise may also occur in infants who present with thin meconium-stained fluid. The hypoxia, hypercapnia, acidosis, and inflammatory reaction associated with meconium aspiration may result in the development of persistent pulmonary hypertension of the newborn (Fig. 50-3 and Chapter 61).
 CLINICAL FEATURES AND DIAGNOSIS
CLINICAL FEATURES AND DIAGNOSIS
The predelivery course may indicate fetal distress with asphyxia, and after delivery, the infant often will have low Apgar scores and require resuscitation. The skin, nails, and umbilical cord may be stained with meconium. Meconium may be in the mouth and pharynx and may be suctioned from the trachea. The infant often will have signs of respiratory distress, such as tachypnea, retractions, and cyanosis. The chest may appear barrel-shaped on examination secondary to lung overinflation. Breath sounds may be obscured by coarse bronchial sounds, and expiration may be prolonged because of small airway obstruction. Occasionally, infants with meconium aspiration syndrome may have minimal symptomatology or even be asymptomatic.
The radiographic findings of meconium aspiration syndrome (MAS) do not always correlate with the severity of the clinical disease. With severe aspiration, the chest radiograph may reveal areas of patchy infiltrates with regions of atelectasis and overinflation with flattened diaphragms (eFig. 50.2  ). Pneumomediastinum and pneumothorax occur in as many as 10% to 15% of infants with MAS23 and may be already present on initial radiograph or develop later in the course of the disease. Early in the clinical course of MAS, hypoxemia and some degree of metabolic acidosis may represent perinatal asphyxia. Later in the course of the disease, the partial pressure of carbon dioxide in arterial blood (PaCO2) may increase and the hypoxia may worsen, reflecting the effects of meconium in the smaller airways, the ongoing inflammatory process, and the surfactant inactivation.
). Pneumomediastinum and pneumothorax occur in as many as 10% to 15% of infants with MAS23 and may be already present on initial radiograph or develop later in the course of the disease. Early in the clinical course of MAS, hypoxemia and some degree of metabolic acidosis may represent perinatal asphyxia. Later in the course of the disease, the partial pressure of carbon dioxide in arterial blood (PaCO2) may increase and the hypoxia may worsen, reflecting the effects of meconium in the smaller airways, the ongoing inflammatory process, and the surfactant inactivation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


