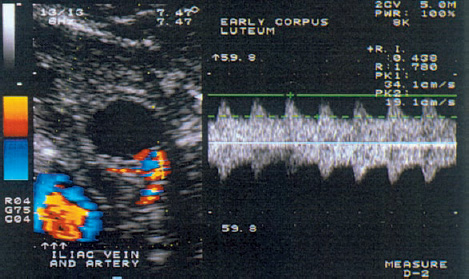
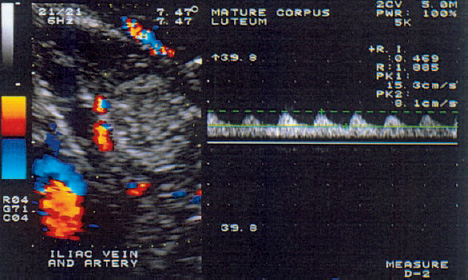
8 Abnormalities of Corpus luteum Function Many authors have described the clinical importance of normal corpus luteum function in the initiation of a normal pregnancy12. An inadequate luteal phase is defined as a delay of more than two days in the histological development of the endometrium relative to the calculated date. It is often a direct result of hormonal dysfunction of the corpus luteum. This dysfunction can have a variety of causes, most notably decreased levels of follicle-stimulating hormone (FSH) in the follicular phase, inadequate secretion of luteinizing hormone (LH), decreased LH and FSH levels at the time of ovulation, and poor endometrial responsiveness to progesterone. Cell types and hormone production. The differentiation of the corpus luteum is an important event in the ovarian cycle and the critical factor in sustaining an early pregnancy. After ovulation has occurred, vessels sprout from the theca to form a network of blood vessels, marking the start of corpus luteum formation (Fig. 8.1). Ultimately the corpus luteum contains various cells: K cells in addition to large and small luteal cells. The large luteal cells develop from the granulosa cells, and the small luteal cells from the thecal cells. The large luteal cells produce more progesterone than the small luteal cells, but the latter appear to be more sensitive to stimulation by LH and chorionic gonadotropin. Also, the small luteal cells appear to produce various angiogenesis factors, and this may occur independently of the production of steroid hormones. Production of the prostaglandins I2, E2, and F2α has been demonstrated in cell cultures. The prostaglandins, whose formation is influenced by lipoxygenase products of arachidonic acid such as 5-HETE (hydroxyeicosatetraenoic acid) and is independent of chorionic gonadotropin, act directly on progesterone metabolism. Prostaglandin I2 and prostaglandin E2 promote progesterone formation, while prostaglandin F2α has a luteolytic action. Besides being regulated by the hypothalamic–pituitary axis (FSH, LH), the corpus luteum has its own paracrine regulatory mechanism whose details require further investigation. Luteal phase. The luteal phase begins with the release of the oocyte and the formation of the corpus luteum, accompanied by a significant rise of LH and FSH. The small luteal cells increasingly produce LH receptors, which stimulate progesterone production. The midluteal phase is characterized by peak levels of circulating LH and progesterone and by the lowest resistance index (RI) in the corpus luteum blood vessels, as Kupesic et al.11 demonstrated by transvaginal pulsed color Doppler sonography (Fig. 8.2). The rising progesterone concentration finally suppresses the secretion of gonadotropins, the LH and progesterone levels fall, and the RI in the luteal blood vessels increases. A condition known as luteal phase defect (LPD) can result from faulty “internal” regulation as well as adverse external factors (e.g., strenuous exercise or ovulation-stimulating medications). Fig. 8.1 Transvaginal ultrasound scan of the ruptured follicle (left). An increased blood flow velocity and decreased RI (0.44) are typical signs that indicate ovulation and the start of corpus luteum formation. Fig. 8.2 Increased blood flow in the mature corpus luteum (left). The Doppler trace indicates a high blood flow velocity and low RI (0.47). Definition. Various terms have been applied to this disorder: luteal phase defect, luteal phase deficiency, short luteal phase, luteal insufficiency, and inadequate luteal phase. All these terms describe the same condition, which consists of a progesterone deficiency, a luteal phase shorter than 11 days, and a delay of 2 or more days in the secretory transformation of the endometrium. Effect of LH. Zeleznik and Little-Ihrig studied the effect of LH on corpus luteum function in rhesus monkeys20. Gonadotropin-releasing hormone (GnRH) was administered to induce gonadotropin secretion. Various plasma LH concentrations were measured, depending on the amount of GnRH administered. It was found that an LH concentration of 50% normal was still able to sustain progesterone secretion during the late luteal phase. These results confirm the hypothesis that regression of the corpus luteum in the nonfertile cycle is due primarily to a reduced luteal cell responsiveness to LH rather than a reduction in gonadotropin secretion. Jones showed that an imbalance between the FSH and LH levels is responsible for inadequate folliculogenesis and for inadequate transformation of the granulosa and theca cells into the granulosa luteal cells and theca luteal cells of the corpus luteum10. This leads to luteal phase defect. Corpus luteum dysfunction with a normal length of the luteal phase may result from impaired granulosa cell function or from an inadequate LH surge with a fairly normal total LH secretion and theca cell response. A short luteal phase is associated with a poor LH surge and low LH secretion. There appears to be a critical LH level that must be maintained after ovulation to ensure the morphological and functional transformation of the granulosa and theca cells and the induction of enzymes for the steroidogenesis of regulatory peptides and various peptide receptors. Strenuous exercise. Beitinis et al.2 studied 28 female students with regular cycles who performed regular, strenuous physical exercise during a two-month training program. All subjects collected daily overnight urine samples for three months: at the start of the study, during a control cycle without exercise, and during two exercise cycles. LH, FSH, estriol, and free progesterone were determined and related to creatinine excretion. Twenty cycles with a luteal phase defect were observed in 18 participants. Four women had an inadequate luteal phase in the first month of the training program, combined with decreased free progesterone secretion and a luteal phase shorter than nine days. During the second exercise month, two inadequate and four short luteal phases were observed. Because disturbances of LH and estriol secretion were also observed in women with short luteal phases, it may be assumed that regular, strenuous exercise, which was associated with significant weight loss in 12 cases, can indeed lead to anovulation. It is interesting to note, however, that only two of the women had a luteal phase defect in both cycles. These results show that physical exercise, change of living conditions, stress, or other extraneous factors can cause menstrual disturbances that may affect the entire cycle or only the luteal phase. It appears, however, that these effects are transient in the majority of cases. Clinical practice has shown that many women will show menstrual abnormalities when followed for several months, but that very few of these problems are permanent. Ovarian stimulation. There is still disagreement whether ovarian stimulation causes luteal insufficiency. Hecht et al.8 showed that luteal insufficiency is rare in clomiphene-induced cycles. On the other hand, Reshef et al.15 found that in 30 women who had been treated with gonadotropin and hCG, 27% of the patients showed inadequate endometrial development. Endometrial biopsy. One of the greatest problems for scientists and clinicians is the detection of luteal insufficiency. One option is the histological analysis of endometrial biopsies. This method is fairly precise, since the amount of progesterone produced by the corpus luteum can be estimated by the transformation that the endometrium has undergone for implantation. In a regular cycle, the biopsy is taken shortly before the start of menstrual bleeding on the basis of an assumed cycle length of 28 days and a luteal phase of 14 days duration. Because the luteal phase may last from 12 to 14 days, however, the optimum timing of the biopsy is disputed. Biopsies that are taken in the early and midluteal phase show greater histological variation than biopsies from the late luteal phase. Timing the biopsy close to menstruation will best reflect the cumulative progesterone activity. A major problem is different biopsy interpretations rendered by different evaluators or by the same evaluator at different times. Gibson et al.5 obtained duplicate endometrial biopsy samples from 25 women in one sitting. Five colleagues evaluated the two biopsy series on two separate occasions at least two weeks apart. In 43.1% of the cases, the same evaluator gave exactly the same reading for duplicate slides from the same patient, and in 5% of cases the same evaluator saw a difference in endometrial transformation of three or more days in duplicate readings. Even greater inconsistencies occurred among different evaluators. The readings agreed in only 25% of the cases, and in 22% of cases the discrepancy was greater than two days. Serum progesterone level. The serum progesterone level is also used in the diagnosis of luteal insufficiency. Minimum levels of 2.5–5.0 ng/ml in the midluteal phase indicate ovulation, while levels of 10–15 ng/ml in this phase correspond to normal corpus luteum function19. But because diurnal variations in progesterone secretion can cause deviations of more than 30% from the mean value, a single progesterone determination does not reflect either corpus luteum function or its effect on the endometrium. Placental protein. A “placental protein 14” has been described, which is expressed by the endometrial glands and has occasionally been detected in the blood of women with anovulatory cycles4. A test procedure has not yet been established. Dawood3 states that the combined use of all test procedures is necessary in order to draw relevant conclusions. Stimulation with clomiphene and hCG.
Morphology and Biochemistry of the Corpus luteum
Conventional Methods in the Diagnosis and Treatment of Luteal Phase Defect
Possible Causes of Luteal Phase Defect
Diagnosis of Luteal Phase Defect
Treatment of Luteal Phase Defect
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree