Background
Premature cervical remodeling resulting in spontaneous preterm birth may begin with premature failure or relaxation at the internal os (termed “funneling”). To date, we do not understand why the internal os fails or why funneling occurs in some cases of premature cervical remodeling. Although the human cervix is thought to be mostly collagen with minimal cellular content, cervical smooth muscle cells are present in the cervix and can cause cervical tissue contractility.
Objective
To understand why the internal os relaxes or why funneling occurs in some cases of premature cervical remodeling, we sought to evaluate cervical smooth muscle cell content and distribution throughout human cervix and correlate if cervical smooth muscle organization influences regional cervical tissue contractility.
Study Design
Using institutional review board–approved protocols, nonpregnant women <50 years old undergoing hysterectomy for benign indications were consented. Cervical tissue from the internal and external os were immunostained for smooth muscle cell markers (α-smooth muscle actin, smooth muscle protein 22 calponin) and contraction-associated proteins (connexin 43, cyclooxygenase-2, oxytocin receptor). To evaluate cervical smooth muscle cell morphology throughout the entire cervix, whole cervical slices were obtained from the internal os, midcervix, and external os and immunostained with smooth muscle actin. To correlate tissue structure with function, whole slices from the internal and external os were stimulated to contract with 1 μmol/L of oxytocin in organ baths. In separate samples, we tested if the cervix responds to a common tocolytic, nifedipine. Cervical slices from the internal os were treated with oxytocin alone or oxytocin + increasing doses of nifedipine to generate a dose response and half maximal inhibitory concentration. Student t test was used where appropriate.
Results
Cervical tissue was collected from 41 women. Immunohistochemistry showed cervical smooth muscle cells at the internal and external os expressed mature smooth muscle cell markers and contraction-associated proteins. The cervix exhibited a gradient of cervical smooth muscle cells. The area of the internal os contained 50-60% cervical smooth muscle cells that were circumferentially organized in the periphery of the stroma, which may resemble a sphincter-like pattern. The external os contained approximately 10% cervical smooth muscle cells that were randomly scattered in the tissue. In organ bath studies, oxytocin stimulated the internal os to contract with more than double the force of the external os (1341 ± 693 vs 523 ± 536 integrated grams × seconds, respectively, P = .009). Nifedipine significantly decreased cervical tissue muscle force compared to timed vehicle control (oxytocin alone) at doses of 10 –5 mol/L (vehicle 47% ± 15% vs oxytocin + nifedipine 24% ± 16%, P = .007), 10 –4 mol/L (vehicle 46% ± 16% vs oxytocin + nifedipine –4% ± 20%, P = .003), and 10 –3 mol/L (vehicle 42% ± 14% vs oxytocin + nifedipine –15% ± 18%, P = .0006). The half maximal inhibitory concentration for nifedipine was 1.35 × 10 –5 mol/L.
Conclusion
Our findings suggest a new paradigm for cervical tissue morphology–one that includes the possibility of a specialized sphincter at the internal os. This new paradigm introduces novel avenues to further investigate potential mechanisms of normal and premature cervical remodeling.
Introduction
Spontaneous preterm birth (sPTB) is a significant obstetric dilemma affecting approximately 10% of US pregnancies. Etiologies vary, but sPTB must eventually involve premature remodeling and dilation of the cervix to allow for delivery of the premature fetus. Although the pathophysiology of premature cervical remodeling is not fully understood, sonographic findings and computational modeling suggest that in some cases, the process starts with dilation of the internal os (the top aspect of the cervix where the uterine arteries insert into the uterus), which is clinically termed “funneling” ( Figure 1 ). Clinicians also describe a “dynamic cervix” where the cervix appears shortened in the absence of uterine contractions, which can be seen if transvaginal ultrasound is performed for several minutes. A dynamic cervix has also been described as cervical shortening in response to fundal pressure. Despite these clinical findings, we still cannot explain why the internal os weakens first in some cases of premature cervical remodeling. Our overall goal is to study whether premature cervical failure at the level of the internal os is due to regional differences in cervical tissue morphology and function.
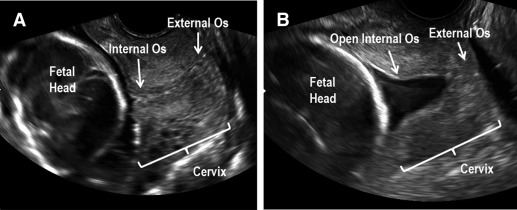
Since the 1940s, the cervix has been characterized as a mostly collagenous structure (90% collagen/extracellular matrix [ECM]) with minimal cellular content (10% smooth muscle cells [SMC]). However, these early studies suffered from technical limitations of the time and used immunohistochemical methods (Masson trichrome staining and subjective evaluation of SMC morphology) that do not specifically identify SMC. Decades later, investigators questioned why SMC exist in the cervix. Interestingly, Bryman et al found that adrenoreceptor agonists and oxytocin stimulate human tissue from the external os (lowermost aspect of the cervix closest to the vagina) to contract, suggesting SMC play a role in cervical tissue contractility.
Since the turn of this century, however, cervical SMC (CSMC) have been largely ignored since CSMC content was thought to be minimal. Instead, researchers focused on identifying alterations in the human cervical collagen network to explain premature cervical failure. Since clinical observation demonstrates the area of the internal os can funnel first and/or is dynamic in some cases of premature cervical remodeling, we believe that the working paradigm of cervical tissue architecture needs to be reevaluated. Here, we use improved immunohistochemical techniques and functional studies to determine if regional differences in CSMC content and distribution influence cervical tissue function. The knowledge from this study will expand our understanding of cervical tissue characteristics that may contribute to normal and abnormal cervical function in pregnancy.
Materials and Methods
Tissue collection
This study was approved by the institutional review board at Columbia University Medical Center and Intermountain Healthcare. Nonpregnant, premenopausal women (<50 years old) undergoing a total hysterectomy for benign indications were consented. Women with an abnormal pap smear or prior cervical surgery were excluded. Demographic data (age, parity, obstetric history, menstrual phase, body mass index, and race) were collected.
Evaluation of CSMC at the internal and external os
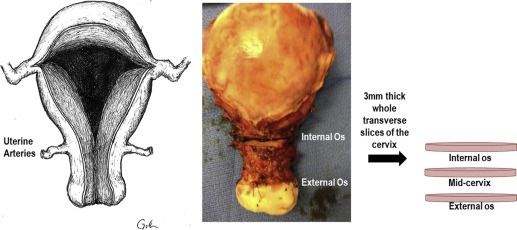
Immediately following hysterectomy, whole transverse slices (3-mm thick) of the cervix were obtained at the internal os and the external os ( Figure 2 ). The slices were fixed in 10% formalin for 24 hours, transferred to 70% ethanol, and paraffin embedded. After normal pathology was confirmed, 5-μm sections were stained with Movat pentachrome to appreciate general tissue structure (collagen, mucin, muscle distribution). To visualize the entire cervical slice, ×10 images from the Movat Pentachrome–stained sections were collected and digitally reconstructed with ZEN software (Zeiss, Thornwood, NY).
Since previous studies that established cervical tissue morphology were technically limited to immunohistochemical methods that could not identify mature, contractile SMC, we initially sought to determine if CSMC at the internal and external os expressed both mature SMC markers and contraction-associated proteins (CAPs). Paraffin-embedded human myometrial tissue obtained from a hysterectomy specimen was used as a positive control. The 5-μm sections of myometrial and cervical tissue (from the internal and external os) were incubated at 40°C overnight, paraffin melted at 60°C for 20 minutes, and then rehydrated using descending ethanol dilutions. Heat-mediated antigen retrieval was performed for 20 minutes using Dako target retrieval solution (Dako, Carpinteria, CA) and colorimetric or immunofluorescent immunohistochemistry (IHC) was performed.
Colorimetric IHC
Tissue sections were incubated in 0.3% hydrogen peroxide for 20 minutes at room temperature to block endogenous peroxidase. Endogenous avidin and biotin were blocked using an avidin/biotin blocking kit (Vector Laboratories, Burlingame, CA). Slides were incubated in blocking solution (phosphate-buffered saline containing 3% bovine serum albumin and 2% goat serum; both Sigma, St Louis, MO) for 1 hour at room temperature. Primary antibodies for commonly used mature SMC markers (α-smooth muscle actin [SMA], SM22, calponin) and CAPs (oxytocin receptor, cyclooxygenase-2) were incubated overnight at 4°C. Primary antibody information is listed in Table 1 . Incubation with biotinylated goat antirabbit secondary antibody (1:500; Vector Laboratories) was performed for 30 minutes at room temperature followed by incubation with avidin and horseradish-peroxidase conjugated biotin in phosphate-buffered saline (Vector Laboratories). Color reaction was performed using diaminobenzidine tetrahydrochloride and the peroxidase substrate (Vector Laboratories). Tissues were counterstained with hematoxylin (Fisher Scientific, Pittsburgh, PA). Imaging was performed on an AxioObserver Z.1 microscope (Zeiss) using an Axiocam ICc camera (Zeiss).
| Antibody | Vendor/catalog no. | Dilution | Description |
|---|---|---|---|
| Smooth muscle cell markers | |||
| α-Smooth muscle actin | Abcam/5694 | 1:100 | Rabbit polyclonal |
| SM22 | Abcam/14106 | 1:200 | Rabbit polyclonal |
| Calponin | Thermo Scientific/EP798Y | 1:200 | Rabbit monoclonal |
| Contraction-associated proteins | |||
| Oxytocin receptor | Sigma/04389 | 1:25 | Rabbit polyclonal |
| Cyclooxygenase-2 | Abcam/52237 | 20 μg/mL | Rabbit polyclonal |
| Connexin 43 | Abcam/11370 | 1:100 | Rabbit polyclonal |
Immunofluorescent IHC
Immunofluorescent IHC was used to assess connexin 43 (gap junction protein) expression as our experience with the antibody for connexin 43 showed optimal staining with this technique. After heat-mediated antigen retrieval (Dako target retrieval solution, Dako), enzyme-mediated antigen retrieval (pepsin 1 mg/mL, [Sigma] in 0.01 N hydrogen chloride) was performed for 30 minutes at 37°C. Sections were incubated in blocking solution (detailed above) for 1 hour at room temperature. Primary connexin 43 antibody (detailed information listed in Table 1 ) was incubated overnight at 4°C and then incubated with goat antirabbit Alexa Fluor 594 secondary antibody for 30 minutes at room temperature (1:200; Invitrogen, Carlsbad, CA). 4′,6-Diamidino-2-phenylindole dihydrochloride (Sigma) was used to stain nuclei. Images were obtained on Eclipse E 800 microscope (Nikon Inc, Melville, NY). For negative controls (colorimetric and immunofluorescent IHC studies) primary antibody was omitted and tissue was incubated in blocking solution overnight.
Evaluation of CSMC architecture in the entire cervix
To investigate CSMC if morphology changed from the proximal to distal end of the cervix, 3-mm whole transverse slices were obtained from the internal os, midcervix (the midpoint between the internal and external os), and external os immediately after hysterectomy ( Figure 2 ). Slices were fixed in 10% formalin for 24 hours, transferred to 70% ethanol, and paraffin embedded. After normal pathology was confirmed, 5-μm sections of each slice were immunostained with SMA (outlined above). To appreciate CSMC organization in each slice, ×10 images were obtained on an AxioObserver Z.1 microscope (Zeiss) and Axiocam ICc camera. Images were then digitally reconstructed with ZEN software (Zeiss). To approximate CSMC content at the internal os, midcervix, and external os, Photoshop CS5 (Adobe Systems Inc., San Jose, CA). 1 was used to count the number of SMA-positive pixels in the tiled images at the internal and external os. Specifically, the number of SMA-positive pixels was divided by the total number of pixels in the cervical slice to obtain the percent of positive SMA pixels in the entire cervical slice.
Organ bath studies
Evaluating regional cervical tissue contractility in response to oxytocin
To evaluate how CSMC content and distribution influences regional cervical tissue contractility, organ bath studies were performed using an additional set of cervical tissue slices collected immediately following hysterectomy. The 3-mm whole transverse cervical tissue slices (from the internal and external os) were immediately placed in ice-cold SmBMII media with the recommended additives (Lonza, Walkersville, MD). For each slice, one end of the tissue slice was fixed to the bottom of 16 mL water-jacketed (37°C) organ bath (Radnoti Glass Technology, Monrovia, CA) and the opposite end was attached to a Grass force transducer (Grass-Telefactor, West Warwick, RI) coupled to Biopac hardware (Biopac Systems Inc, Goleta, CA). Acknowledge 7.3.3 software (Biopac Systems Inc) was used for continuous digital recording of muscle force. Baths contained a modified Krebs−Henseleit buffer (115.0 mmol/L sodium chloride, 2.5 mmol/L potassium chloride, 1.9 mmol/L calcium chloride, 2.5 mmol/L magnesium sulfate, 25 mmol/L sodium bicarbonate, 1.4 mmol/L sodium dihydrogen phosphate, 5.6 mmol/L D-glucose) that was continuously bubbled with 95% oxygen/5% carbon dioxide. Tissues were equilibrated to 1.0 g of isometric tension for 1 hour (with buffer replacement every 20 minutes) and then stimulated to contract using 1 μmol/L oxytocin (Sigma). Muscle force was analyzed for 20 minutes. Prizm GraphPad software (GraphPad Software Inc, La Jolla, CA) and Student t test were used to compare mean amplitude of force between the internal and external os slices.
Evaluating if nifedipine tocolyzes the internal os
In a subset of the women enrolled for the oxytocin studies detailed above, an additional 3-mm whole transverse cervical tissue slice was obtained from the internal os. After similar preparation and equilibration, 1 internal os slice from each patient was stimulated to contract using 1 μmol/L oxytocin alone (as outlined above) to assess for expected time-dependent reductions in contractility after oxytocin exposure. The adjacent slice from the internal os was treated with 1 μmol/L oxytocin followed by increasing doses of nifedipine (10 –9 to 10 –3 mol/L, Sigma) every 15 minutes to generate a dose response and IC 50 , which was calculated using Prizm GraphPad software (GraphPad Software Inc). Nifedipine-mediated effects on oxytocin-induced contractility were expressed as a percentage change from the initial contraction before nifedipine was added. To calculate the effect of nifedipine at each dose/time point, the percent remaining force contraction in the nifedipine samples were normalized to the appropriately timed percentage of the oxytocin-induced contraction in our control (oxytocin only) samples. Student t test was used to compare the percent remaining cervical tissue muscle force at each time point in the oxytocin + nifedipine vs timed vehicle control (oxytocin alone) samples.
Materials and Methods
Tissue collection
This study was approved by the institutional review board at Columbia University Medical Center and Intermountain Healthcare. Nonpregnant, premenopausal women (<50 years old) undergoing a total hysterectomy for benign indications were consented. Women with an abnormal pap smear or prior cervical surgery were excluded. Demographic data (age, parity, obstetric history, menstrual phase, body mass index, and race) were collected.
Evaluation of CSMC at the internal and external os
Immediately following hysterectomy, whole transverse slices (3-mm thick) of the cervix were obtained at the internal os and the external os ( Figure 2 ). The slices were fixed in 10% formalin for 24 hours, transferred to 70% ethanol, and paraffin embedded. After normal pathology was confirmed, 5-μm sections were stained with Movat pentachrome to appreciate general tissue structure (collagen, mucin, muscle distribution). To visualize the entire cervical slice, ×10 images from the Movat Pentachrome–stained sections were collected and digitally reconstructed with ZEN software (Zeiss, Thornwood, NY).
Since previous studies that established cervical tissue morphology were technically limited to immunohistochemical methods that could not identify mature, contractile SMC, we initially sought to determine if CSMC at the internal and external os expressed both mature SMC markers and contraction-associated proteins (CAPs). Paraffin-embedded human myometrial tissue obtained from a hysterectomy specimen was used as a positive control. The 5-μm sections of myometrial and cervical tissue (from the internal and external os) were incubated at 40°C overnight, paraffin melted at 60°C for 20 minutes, and then rehydrated using descending ethanol dilutions. Heat-mediated antigen retrieval was performed for 20 minutes using Dako target retrieval solution (Dako, Carpinteria, CA) and colorimetric or immunofluorescent immunohistochemistry (IHC) was performed.
Colorimetric IHC
Tissue sections were incubated in 0.3% hydrogen peroxide for 20 minutes at room temperature to block endogenous peroxidase. Endogenous avidin and biotin were blocked using an avidin/biotin blocking kit (Vector Laboratories, Burlingame, CA). Slides were incubated in blocking solution (phosphate-buffered saline containing 3% bovine serum albumin and 2% goat serum; both Sigma, St Louis, MO) for 1 hour at room temperature. Primary antibodies for commonly used mature SMC markers (α-smooth muscle actin [SMA], SM22, calponin) and CAPs (oxytocin receptor, cyclooxygenase-2) were incubated overnight at 4°C. Primary antibody information is listed in Table 1 . Incubation with biotinylated goat antirabbit secondary antibody (1:500; Vector Laboratories) was performed for 30 minutes at room temperature followed by incubation with avidin and horseradish-peroxidase conjugated biotin in phosphate-buffered saline (Vector Laboratories). Color reaction was performed using diaminobenzidine tetrahydrochloride and the peroxidase substrate (Vector Laboratories). Tissues were counterstained with hematoxylin (Fisher Scientific, Pittsburgh, PA). Imaging was performed on an AxioObserver Z.1 microscope (Zeiss) using an Axiocam ICc camera (Zeiss).
| Antibody | Vendor/catalog no. | Dilution | Description |
|---|---|---|---|
| Smooth muscle cell markers | |||
| α-Smooth muscle actin | Abcam/5694 | 1:100 | Rabbit polyclonal |
| SM22 | Abcam/14106 | 1:200 | Rabbit polyclonal |
| Calponin | Thermo Scientific/EP798Y | 1:200 | Rabbit monoclonal |
| Contraction-associated proteins | |||
| Oxytocin receptor | Sigma/04389 | 1:25 | Rabbit polyclonal |
| Cyclooxygenase-2 | Abcam/52237 | 20 μg/mL | Rabbit polyclonal |
| Connexin 43 | Abcam/11370 | 1:100 | Rabbit polyclonal |
Immunofluorescent IHC
Immunofluorescent IHC was used to assess connexin 43 (gap junction protein) expression as our experience with the antibody for connexin 43 showed optimal staining with this technique. After heat-mediated antigen retrieval (Dako target retrieval solution, Dako), enzyme-mediated antigen retrieval (pepsin 1 mg/mL, [Sigma] in 0.01 N hydrogen chloride) was performed for 30 minutes at 37°C. Sections were incubated in blocking solution (detailed above) for 1 hour at room temperature. Primary connexin 43 antibody (detailed information listed in Table 1 ) was incubated overnight at 4°C and then incubated with goat antirabbit Alexa Fluor 594 secondary antibody for 30 minutes at room temperature (1:200; Invitrogen, Carlsbad, CA). 4′,6-Diamidino-2-phenylindole dihydrochloride (Sigma) was used to stain nuclei. Images were obtained on Eclipse E 800 microscope (Nikon Inc, Melville, NY). For negative controls (colorimetric and immunofluorescent IHC studies) primary antibody was omitted and tissue was incubated in blocking solution overnight.
Evaluation of CSMC architecture in the entire cervix
To investigate CSMC if morphology changed from the proximal to distal end of the cervix, 3-mm whole transverse slices were obtained from the internal os, midcervix (the midpoint between the internal and external os), and external os immediately after hysterectomy ( Figure 2 ). Slices were fixed in 10% formalin for 24 hours, transferred to 70% ethanol, and paraffin embedded. After normal pathology was confirmed, 5-μm sections of each slice were immunostained with SMA (outlined above). To appreciate CSMC organization in each slice, ×10 images were obtained on an AxioObserver Z.1 microscope (Zeiss) and Axiocam ICc camera. Images were then digitally reconstructed with ZEN software (Zeiss). To approximate CSMC content at the internal os, midcervix, and external os, Photoshop CS5 (Adobe Systems Inc., San Jose, CA). 1 was used to count the number of SMA-positive pixels in the tiled images at the internal and external os. Specifically, the number of SMA-positive pixels was divided by the total number of pixels in the cervical slice to obtain the percent of positive SMA pixels in the entire cervical slice.
Organ bath studies
Evaluating regional cervical tissue contractility in response to oxytocin
To evaluate how CSMC content and distribution influences regional cervical tissue contractility, organ bath studies were performed using an additional set of cervical tissue slices collected immediately following hysterectomy. The 3-mm whole transverse cervical tissue slices (from the internal and external os) were immediately placed in ice-cold SmBMII media with the recommended additives (Lonza, Walkersville, MD). For each slice, one end of the tissue slice was fixed to the bottom of 16 mL water-jacketed (37°C) organ bath (Radnoti Glass Technology, Monrovia, CA) and the opposite end was attached to a Grass force transducer (Grass-Telefactor, West Warwick, RI) coupled to Biopac hardware (Biopac Systems Inc, Goleta, CA). Acknowledge 7.3.3 software (Biopac Systems Inc) was used for continuous digital recording of muscle force. Baths contained a modified Krebs−Henseleit buffer (115.0 mmol/L sodium chloride, 2.5 mmol/L potassium chloride, 1.9 mmol/L calcium chloride, 2.5 mmol/L magnesium sulfate, 25 mmol/L sodium bicarbonate, 1.4 mmol/L sodium dihydrogen phosphate, 5.6 mmol/L D-glucose) that was continuously bubbled with 95% oxygen/5% carbon dioxide. Tissues were equilibrated to 1.0 g of isometric tension for 1 hour (with buffer replacement every 20 minutes) and then stimulated to contract using 1 μmol/L oxytocin (Sigma). Muscle force was analyzed for 20 minutes. Prizm GraphPad software (GraphPad Software Inc, La Jolla, CA) and Student t test were used to compare mean amplitude of force between the internal and external os slices.
Evaluating if nifedipine tocolyzes the internal os
In a subset of the women enrolled for the oxytocin studies detailed above, an additional 3-mm whole transverse cervical tissue slice was obtained from the internal os. After similar preparation and equilibration, 1 internal os slice from each patient was stimulated to contract using 1 μmol/L oxytocin alone (as outlined above) to assess for expected time-dependent reductions in contractility after oxytocin exposure. The adjacent slice from the internal os was treated with 1 μmol/L oxytocin followed by increasing doses of nifedipine (10 –9 to 10 –3 mol/L, Sigma) every 15 minutes to generate a dose response and IC 50 , which was calculated using Prizm GraphPad software (GraphPad Software Inc). Nifedipine-mediated effects on oxytocin-induced contractility were expressed as a percentage change from the initial contraction before nifedipine was added. To calculate the effect of nifedipine at each dose/time point, the percent remaining force contraction in the nifedipine samples were normalized to the appropriately timed percentage of the oxytocin-induced contraction in our control (oxytocin only) samples. Student t test was used to compare the percent remaining cervical tissue muscle force at each time point in the oxytocin + nifedipine vs timed vehicle control (oxytocin alone) samples.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


