- Glucose homeostasis and its abnormalities
- Disorders of calcium, phosphate and magnesium metabolism
- Disorders of magnesium metabolism
- Disorders of sodium and potassium metabolism
- Endocrine gland disorders
- Abnormalities of the adrenal gland
- Inborn errors of metabolism
Introduction
Some endocrine and metabolic disorders present acutely in the newborn period and can be life-threatening. They must be included in the differential diagnosis of any serious or unexplained illness. Prompt diagnosis and treatment is life-saving. In other cases, clinical presentation may not be so acute but if undiagnosed and left untreated, can cause serious problems such as growth failure, developmental delay and poor cognitive (intellectual) development. Neonatal screening for conditions which can have serious consequences, and for which treatment is available, is undertaken in most developed countries. Investigation and treatment of endocrine and metabolic diseases can be complex and requires specialist input. Some are readily and successfully treated whilst for others, specific treatment is not available. Babies with endocrine and metabolic problems require long-term treatment and follow-up by a specialist team.
Glucose Homeostasis and its Abnormalities
The fetus has a continuous supply of glucose from the mother via the placenta, and consequently fetal blood glucose levels are the same as the mother’s. At birth the newborn’s blood glucose rapidly falls to approximately 75% of the maternal blood glucose level and the infant has to switch rapidly to endogenous gluconeogenesis until feeding is established.
Glucose and oxygen are the main metabolic substrates of the mature brain, but in the neonate the brain can use alternative metabolic fuels such as lactate and ketones. Birth at full term is characterized by a vigorous ketogenic response, but this is impaired in preterm infants and infants who experience intrauterine growth restriction (IUGR). This is why the brain can function normally, or near normally, despite very low levels of blood glucose. Profound neurological compromise and irreversible damage occur if the brain is deprived of glucose and alternative metabolic substrates.
Glucose Metabolism
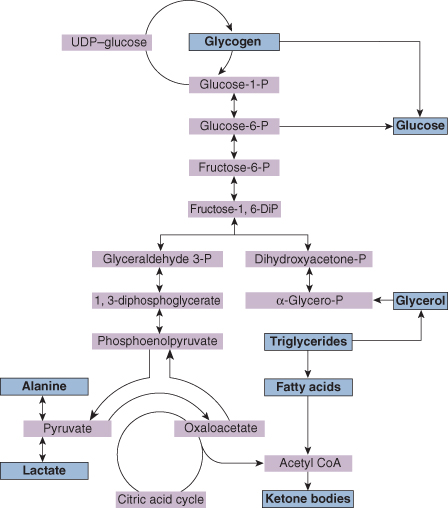
Figure 21.1 summarizes the main metabolic pathways involved in gluconeogenesis.
- Glycogen production and glycogenolysis. These occur largely in the liver and muscles, but only if liver glycogen is available for rapid breakdown to glucose.
- Gluconeogenesis. The most important substrates are amino acids (particularly alanine), lactate, pyruvate and glycerol. The points at which these are metabolized are shown in Fig. 21.1.
- Lipolysis. Glycerol is metabolized from adipose tissue and can be directly utilized in gluconeogenesis metabolism. Other products of lipolysis (fatty acids and triglycerides) are metabolized to ketone bodies, which may be used directly in energy production, particularly by the brain. Ketone body production is stimulated by infant feeding, particularly by breast milk.
These mechanisms are under the control of the endocrine system and are affected by insulin, glucagon, cortisol and growth hormone. Therefore, so that neonates can regulate blood sugar within the physiological range, they must be endowed with adequate liver glycogen, lipid stores and effective metabolic pathways including glycogenolysis and gluconeogenesis, as well as overall endocrine control. Hypoglycaemia will rapidly develop if any of these processes are disturbed.
Measurement of Blood Glucose
It is essential to measure blood glucose rapidly and accurately in high-risk infants, and a variety of techniques have been developed to give cotside values. Glucose concentrations in plasma or serum are 10–15% higher than in whole blood, and many bedside techniques rely on whole-blood methods, whereas the laboratory techniques are more likely to measure serum glucose levels.
Hypoglycaemia
The definition of hypoglycaemia has been gradually refined over the last 30 years and higher values for blood sugar are now accepted as normal than was the case in the past. Hypoglycaemia is defined as a blood sugar concentration falling below a predetermined reference value, but this is not usually helpful in a clinical setting. A more important question is what the lowest acceptable level is in an individual baby before physiological compromise occurs. As discussed above, neonatal cerebral function may continue normally despite very low levels of blood sugar because an alternative energy substrate is available.
Attempts have been made to define the level of blood sugar at which cerebral dysfunction occurs and then to set ‘normoglycaemia’ levels above this. This is based on observational studies in which premature infants (birthweight <1850 g) with blood sugar levels less than 2.6 mmol/L were found to be at increased risk of lower neurodevelopmental scores, particularly when blood sugar values were below this figure on repeated occasions. It has also been shown that a deterioration in neurological function (measured by evoked potentials) occurred with a blood sugar level below 2.6 mmol/L. On the basis of these studies we can define the lower limit of ‘normoglycaemia’ as 2.6 mmol/L although levels below this do not necessarily mean that damage will occur; however, we recommend maintaining the blood sugar of preterm infants and term infants who are at risk of hypoglycaemia (see below) above 2.5 mmol/L.
Who should be Monitored for Hypoglycaemia?
The majority of babies who are prone to hypoglycaemia can be predicted on the basis of easily identifiable risk factors (Box 21.1). Careful monitoring of these babies’ blood sugar will alert the clinician to the onset of hypoglycaemia and enable management aimed to prevent symptomatic hypoglycaemia.
- Infants of diabetic mothers
- Growth-restricted infants
- Premature infants
- Birth asphyxia
- On rewarming after hypothermia
- Infection
- Polycythaemia
- Beckwith–Wiedemann syndrome
In babies at risk of hypoglycaemia an early and frequent feeding regimen should be instituted with regular blood sugar monitoring for the first 24 h. ‘At-risk’ infants should be fed within 2 h of birth. Breast feeding is recommended with a frequent administration of expressed breast milk. Formula and IV dextrose may be needed if early breast milk is insufficient. A suitable regimen would be full-strength formula given 2 hourly, although if the mother is adamant that she wants to feed breast milk only an intravenous 10% glucose infusion can be used. Small for gestational age (SGA) infants often tolerate up to 100 mL/kg on day 1 of life. If asymptomatic hypoglycaemia fails to be corrected by early, frequent feeds, an infusion with 10% dextrose will be necessary.
Blood sugar estimates should be made immediately before the feed, as this is the time when the blood sugar is likely to be at its lowest point. In view of the unreliability of reagent strip tests, it may be preferable to undertake more accurate measurement of blood sugar less frequently: one regimen recommends once or twice daily laboratory measurements starting immediately before the second feed. More frequent measurements are required in the presence of actual hypoglycaemia. When blood sugar levels are satisfactory, the frequency of heel-prick estimations is reduced before ceasing.
Symptoms of Hypoglycaemia
The symptoms of hypoglycaemia in the newborn can be divided into major and minor (see Box 21.2).
- Major. Apnoea, convulsions and coma. Rarely, prolonged hypoglycaemia may cause congestive heart failure or persistent pulmonary hypertension
- Minor. Jitteriness, irritability, tremors, apathy, cyanotic spells and temperature instability
Causes of Hypoglycaemia
These can be considered under five major headings, which are summarized in Table 21.1.
Table 21.1 Causes of neonatal hypoglycaemia
| Decreased substrate availability | SGA infants (see Chapter 12) Premature infants |
| Multiple birth, especially if growth retarded | |
| Increased glucose utilization | Hyperinsulinaemia |
| Infant of a diabetic mother | |
| Rhesus isoimmunization | |
| Nesidioblastosis (hypertrophy of pancreas) | |
| Islet cell tumour | |
| Beckwith–Wiedemann syndrome | |
| Polycythaemia | |
| Inability to utilize glucose | Glycogen storage disease |
| Galactosaemia | |
| Fructose intolerance | |
| Inborn errors of metabolism | |
| Iatrogenic | Inappropriate infusion of glucose |
| Miscellaneous | Birth asphyxia |
| Endocrine deficiencies (e.g. Congenital Adrenal Hyperplasia) | |
| Hypopituitarism |
Management
The major emphasis should be to prevent hypoglycaemia developing, and this is done by recognizing babies who are at higher risk of this condition and giving early and appropriate feeding (see above).
In babies with persistently low glucose level despite feeding, an IV infusion of 10% dextrose at a rate of 80–100 mL/kg per 24 h should be started. If the blood glucose estimate is still less than 2.6 mmol/L (40 mg/100 mL), the dextrose concentration may need to be increased to 15% dextrose. Up to 12.5% dextrose may be infused via a peripheral IV line. If higher concentrations are required, a central IV line will be necessary. Where possible oral feeding should be continued.
Resistant (or Persistent) Hypoglycaemia
Rarely, the above regimen fails to control hypoglycaemia and hyperinsulinaemia should be suspected. Under these circumstances other forms of treatment are necessary to control the hypoglycaemia including:
- hydrocortisone
- glucagon
- diazoxide
- somatostatin analogue SMS-201-995 (useful in the short-term management of neonatal hyperinsulinism)
- laparotomy and subtotal pancreatectomy when an insulinoma is strongly suspected.
Investigations
Hypoglycaemia in most infants resolves spontaneously within a few days, but it is sometimes more severe or fails to resolve rapidly. Hyperinsulinaemia is suspected clinically when a baby with severe non-ketotic hypoglycaemia requires a glucose infusion rate exceeding 10 mg/kg per minute to maintain normoglycaemia. These babies have a very brisk response to glucagon injections, with the blood glucose increasing to >1.7 mmol/L above baseline. The definitive diagnosis of hyperinsulinism is made by measurement of serum insulin (>10 mU/mL) during an episode of hypoglycaemia (<2.2 mmol/L). The plasma should be separated and frozen immediately after taking the sample if reliable results are to be obtained. A screen for inborn errors of metabolism may be necessary in some cases. A detailed ultrasound of abdomen and exploratory laparotomy may be necessary in infants suspected of having islet cell tumours. Inborn errors of metabolism or endocrine problems such as hypopituitarism may rarely cause hypoglycaemia, and if these are considered to be a possible cause, appropriate investigations should be undertaken to diagnose or exclude these conditions.
Prognosis
Severe symptomatic hypoglycaemia carries a very poor prognosis. Approximately half of these babies will die and half of the survivors will have severe neurodevelopmental abnormalities, including cognitive delay, convulsions, generalized hypertonia and microcephaly. The prevention of symptomatic hypoglycaemia is one of the most important factors in preventing brain damage in the whole of neonatal medicine.
It is widely believed that infants with asymptomatic hypoglycaemia are not at risk of adverse neurodevelopmental outcome. However, some studies do suggest that neural dysfunction can occur with blood sugar levels below 2.6 mmol/L, even though the baby may not show clinical symptoms. This argument is unresolved.
Specific Causes of Hypoglycaemia
Infants of Diabetic Mothers
Maternal diabetes is classified as follows:
- pregestational
- type 1: the basic cause is beta-cell destruction
- type 2: this is due to insulin resistance with an insulin secretory defect
- gestational diabetes.
Infants of diabetic mothers (IDMs) have unique problems and require specialized neonatal care. The prognosis for the diabetic pregnancy depends on the severity of the diabetes and the quality of diabetic control during pregnancy.
The two main factors determining whether maternal diabetes will have an effect on the fetus and baby are the vascular complications that the diabetes causes in the mother, and the blood glucose control during pregnancy.
- Vascular disease. Mothers with vascular complications as a result of diabetes are much more likely to develop hypertension in pregnancy, which may affect fetal growth and well-being.
- Glucose control. The outcome of pregnancy in diabetic women also depends on glucose control both before conception and during gestation. Diabetic women should have their diabetes very carefully managed before conception, and combined care through pregnancy by a physician and obstetrician is essential. The blood sugar should be maintained below 8 mmol/L with soluble insulin if necessary, and hypoglycaemia avoided. On this regimen the complications for the fetus are reduced and may be avoided completely. A variety of congenital malformations are particularly common in women with diabetes, and the risk appears to depend on the mother’s prepregnancy blood sugar levels.
Clinical Features of Infants of Diabetic Mothers
Complications to the fetus are likely to occur in diabetic women in whom glucose control has been less than adequate. The frequencies of complications in IDM and in gestational diabetic mothers are given in Table 21.2. Complications include:
- Congenital malformations. The most frequent congenital abnormalities in IDM are:
- congenital heart disease, especially ventricular septal defect, transposition of the great vessels, coarctation of the aorta
- renal vein thrombosis
- sacral and coccygeal agenesis (caudal regression syndrome)
- left microcolon
- hypertrophic cardiomyopathy – this mainly affects the intraventricular septum and may cause ventricular outflow obstruction; it is a transient condition that resolves in the first few months of life; inotropic drugs such as digoxin should be avoided.
- congenital heart disease, especially ventricular septal defect, transposition of the great vessels, coarctation of the aorta
- Stillbirth. There is an increased risk of intrauterine fetal death during pregnancy.
- Infants born to mothers with diabetic vascular disease are more likely to be SGA.
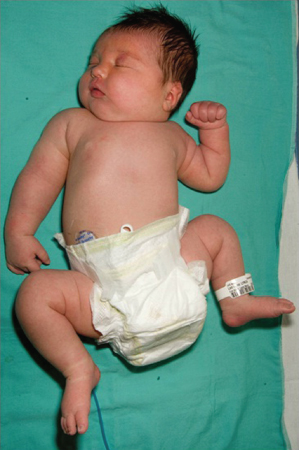
- Macrosomia. Insulin is a major trophic hormone influencing fetal growth, and hyperinsulinaemic fetuses become macrosomic. These infants are plethoric, obese and ‘Cushingoid’ in appearance, and have an enlarged heart, liver and spleen (Fig. 21.2). They have excessive fat stores and inhibition of lipolysis and β-oxidation resulting from hyperinsulinaemia. The large size predisposes to birth-related problems, including:
- Neonatal hypoglycaemia. Chronically elevated maternal glucose levels cause hyperplasia of the islet beta cells in the fetal pancreas with fetal hyperinsulinism. Once the baby is born the high circulating insulin causes neonatal hypoglycaemia lasting for several days. There are three common patterns:
- transient hypoglycaemia, which lasts 1–4 h, followed by a spontaneous rise in the blood sugar
- prolonged initial hypoglycaemia lasting 24–48 h
- rarely there may be a mild initial hypoglycaemia, followed in 12–24 h by more severe hypoglycaemia, which may be symptomatic.
- transient hypoglycaemia, which lasts 1–4 h, followed by a spontaneous rise in the blood sugar
- Insulin has an antagonistic effect on surfactant development and hyperinsulinaemic babies are at much greater risk of developing respiratory distress due to surfactant deficiency, retained lung fluid or polycythaemia, even at full term.
Table 21.2 Frequency of complications (percentages) in infants of diabetic mothers and infants of gestational diabetic mothers. Complications are related to the quality of glucose control in pregnancy
| Complications | IDM (%) | IGDM (%) |
| Uneventful course | 50 | 80 |
| RDS | 30 | 10 |
| Hypoglycaemia (asymptomatic and symptomatic) | 60 | 16 |
| Symptomatic hypoglycaemia | 20 | 10 |
| Hypocalcaemia | 25 | 15 |
| Polycythaemia | 40 | 30 |
| Hyperbilirubinaemia | 50 | 25 |
| Congestive heart failure | 10 | Unknown |
| Congenital abnormalities | 10 | 3 |
IDM, infants of diabetic mothers; IGDM, infants of gestational diabetic mothers; RDS, respiratory distress syndrome.
Figure 21.2 Characteristic appearance of the macrosomic infant of a poorly controlled diabetic mother. Note right sided brachial plexus injury.
Management
Careful control of diabetes during pregnancy decreases many of the complications. Management of the pregnancy involves obsessional diabetic control, planned delivery in a suitably equipped hospital, examination for congenital abnormalities and screening for anticipated complications, especially hypoglycaemia.
Prognosis for Infants of Diabetic Mothers
This depends on the quality of glucose control in pregnancy. Published studies give perinatal mortality rates of about 30/1000 for diabetic pregnancies, but this has improved considerably with counselling during the periconception period and better antenatal surveillance of diabetic mothers.
Congenital Hyperinsulinism
This is due to a group of disorders in the regulatory function of pancreatic beta cells resulting in unregulated secretion of insulin and severe neonatal hypoglycaemia. Specific gene defects have been identified as the cause in 50% of cases. The term nesidioblastosis was previously used to describe congenital hyperinsulinism, but the histological features of it are seen in the normal pancreas and the term is no longer used. Congenital hyperinsulinism should be considered in infants who require glucose infusion exceeding 15 mg/kg/min to prevent hypoglycaemia and confirmed by showing high levels of insulin during hypoglycaemia. Few ketone bodies are produced during the hypoglycaemic episodes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree