- Role of amniotic fluid
- Renal physiology
- Normal urine output
- Investigation of renal disease
- Presentation of renal disease
- Acute renal failure
- Urinary tract infection
- Renal masses
- Cystic disease of the kidneys
- Haematuria
- Congenital abnormalities
Introduction
Many renal disorders are now diagnosed on routine antenatal ultrasound. Some of these conditions are fatal, others require active management and some will need conservative observation only. This chapter discusses the basic physiology of the kidney, its role in amniotic fluid, presentation and investigation of renal disease, and specific conditions which may affect the renal tract.
Role of Amniotic Fluid
Amniotic fluid volume is regulated from a number of fetal pathways including urine production, lung fluid secretion and fetal swallowing. The fetal kidney is the major contributor to the volume of amniotic fluid. Ultimately all amniotic fluid is derived via the placenta. Some of the roles of amniotic fluid are described in Box 18.1.
- Providing space for fetal growth and movement
- Cushioning the umbilical cord against compression
- Protecting the fetus against trauma
- Providing protection against infection
- Maintenance of fetal temperature
- Providing nutrients, hormones and growth factors
- Development of respiratory, gastrointestinal and musculoskeletal systems
Renal Physiology
The first permanent nephrons appear at around 8 weeks’ gestation and nephrogenesis is complete by 36 weeks’ gestation. The increase in renal mass thereafter is predominantly tubular growth. The human has approximately 1 million nephrons. Urine production commences shortly after the first nephrons develop; however, the fetus does not depend on the kidney to excrete waste products as the placenta performs this function. Renal function in the newborn depends on both gestational and postnatal age. Renal function rapidly matures within the first week after birth.
The newborn baby is largely water. The total body water (TBW) is 75% of body weight at term. The TBW is 80–90% in preterm infants of 26–30 weeks’ gestation.
The size of the extracellular fluid compartment (ECF) decreases steadily throughout life from approximately 65% of body weight at 26 weeks to approximately 40% at term and 20% by 10 years of age. Adaptation to the ex utero environment requires rapid loss of excess fluid. The net water and sodium balance is negative in the first few days of life and this is the most important reason for babies losing weight in the first week of life. Normal weight loss can be up to 10% in term babies, and 15% in preterm infants.
Glomerular Filtration Rate
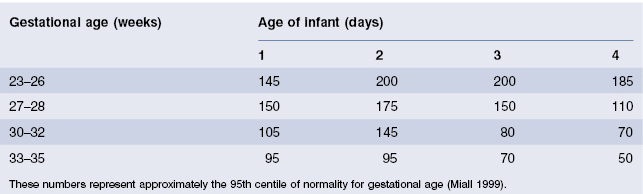
The newborn infant has a very low glomerular filtration rate (GFR) being approximately 20% of adult values. Preterm infants have lower GFR than term infants. After 34 weeks’ gestation, and in response to birth, there is a marked increase in GFR. Although maturation of GFR is most rapid in the first month of life, it continues into the second year. The measurement of serum creatinine is the most convenient index of GFR in infants. Creatinine measured in the first 24 h after birth is not clinically useful as it is more likely to reflect maternal renal function. Plasma urea is also unreliable in neonates as it increases with catabolism even in the presence of normal renal function. The normal limits of creatinine for preterm infants for preterm infants in the first 4 days of life are shown in Table 18.1.
Table 18.1 Upper limit for normal serum creatinine levels (µmol/L) in neonates
Tubular Function
The concentrating ability of the developing kidney increases throughout gestation and improves rapidly after birth. This is due partly to elongation of the collecting tubes and partly to a hormonal effect (see below).
Tubular function can most easily be assessed by measuring the fractional excretion of sodium (FES); in the newborn this should be less than 2.5%.
Sodium Conservation
The fetus has a very poor ability to conserve (reabsorb) sodium. In adults 80–90% of filtered sodium is reabsorbed in the proximal convoluted tubule. Sodium potassium adenosine triphosphatase (Na+,K+-ATPase) creates the electrical chemical gradient for transport. Sodium that enters the distal convoluted tube and collecting ducts is reabsorbed under the influence of aldosterone. Premature babies have decreased Na+,K+-ATPase in the proximal tubule and therefore higher sodium loads are delivered to the distal tubules. They also have limited aldosterone responsiveness at the distal tubule (and therefore less sodium absorption). Maturation of the system appears to be increased by the stress of delivery. Birth causes an increase in the Na+,K+-ATPase, upregulation of transporter proteins and increasing responsiveness of the distal tubule to aldosterone.
Hormonal Function
The kidney is influenced by a number of hormones.
Antidiuretic Hormone
Antidiuretic hormone (ADH) increases water reabsorption from the collecting ducts. It is present from early in fetal life but the fetal kidney is relatively insensitive to it. After birth the collecting ducts become more sensitive. ADH is active in very premature infants, and even the most immature infant is capable of concentrating the urine to a remarkable extent within days of birth.
Renin–Aldosterone
Renin levels are higher in newborn infants than in adults and increase in response to sodium loss. However, the adrenal does not respond with high aldosterone levels and consequently sodium retention is poor, but matures in response to birth.
Normal Urine Output
Due to the reduced renal concentrating ability the maximum flow rate is 300 mL/kg per day and the minimum is 25 mL/kg per day. Over 90% of normal infants pass urine in the first 24 h of life, and 98% have voided by 48 h from birth. On day 1 of life, the urine output is approximately 0.5 mL/kg per hour, thereafter it is 2–3 mL/kg per hour. Neonates often do not completely empty their bladder on voiding.
- Haemolysis can falsely elevate (especially if collected via heel prick)
- Tissue serum (particularly in oedematous patients) can also falsely elevate
- Delay in processing can elevate.
Investigation of Renal Disease
Ultrasound
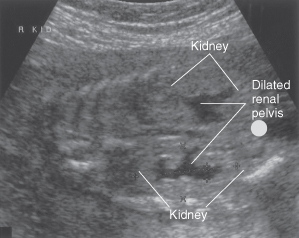
This is the mainstay of investigating the structure of the renal tract both before and after birth (Fig. 18.1). Renal Doppler can be used to assess blood flow in and out of the kidney.
Figure 18.1 Longitudinal ultrasound view of fetal abdomen showing bilateral renal pelvocalyceal dilatation.
Courtesy of Dr R Cincotta.
MAG 3 Renogram
A MAG 3 (mercaptoacetyltriglycine) renogram is a kinetic scan that involves intravenous injection of a radioactive tracer, which is taken up by the kidney thereby enabling excretion curves to be plotted and obstruction to be visualized. Delay in excretion of isotopes from the kidney is suggestive of obstructive uropathy such as pelvi-ureteric junction (PUJ) obstruction. It is also possible to estimate differential kidney function using this scan prior to the excretion phase.
DMSA Scan
A dimercaptosuccinic acid (DMSA) radionuclide scan is a static scan that delineates renal scarring or dysplasia and allows better estimation of differential renal function. It may show false-positive results if done during or soon after an episode of acute urinary tract infection.
Micturating Cystourethrogram
The micturating cystourethrogram (MCUG) is the investigation of choice for vesicoureteric reflux. It involves urethral catheterization with the injection of radio-opaque dye into the bladder and observation of whether the dye refluxes into the ureters on micturition. It is an invasive procedure and prophylactic antibiotics are often recommended before and after the procedure.
Presentation of Renal Disease
Most significant structural renal anomalies are detected antenatally as the result of routine fetal anomaly scanning. Some diagnoses are so severe (e.g. renal agenesis) that termination of pregnancy or palliative care may be offered to the parents. In other cases the neonatal team will be notified before delivery of a baby with a renal anomaly and a variety of investigations will be required after birth. Genitourinary disease in the newborn may present in a number of different ways. These are discussed separately.
Potter’s Syndrome
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


